玉米是重要的粮食作物,玉米的产量潜力受遗传、环境和栽培措施等影响,栽培措施如玉米种植化控技术的研究和应用对玉米生长发育和产量形成具有重要的作用和显著增产效果[1-6]。γ-氨基丁酸(GABA)参与植物碳/氮平衡调控[7-9],增强植物的抗逆生理[10-12],影响植株的生长和发育、植物的形态建成、植株物质积累以及作物的产量形成等生理生化代谢过程[13-16]。前期研究表明,γ-氨基丁酸(GABA)可调控玉米幼苗的光合作用、氮代谢和光合物质积累[16]。在大田试验条件下,外源喷施GABA对糯玉米的生长发育、叶片光合生理特性和糯玉米品种的产量形成的影响研究报道较少。在田间试验条件下,以熟知的糯玉米品种为材料,研究根际浇灌外源γ-氨基丁酸(GABA)对糯玉米品种生长、光合和产量形成的影响,以期探明γ-氨基丁酸(GABA)对糯玉米产量形成影响及其光合生理。研究将为糯玉米产量提高化控技术应用提供指导。
1 材料和方法
1.1 试验设计
试验于2020年秋季在广东省农业科学院白云试验基地进行。以糯玉米品种粤彩糯2号(V1)、京科糯928(V2)和粤白甜糯7号(V3)为供试试材,京科糯928的种子由北京农林科学院玉米中心提供,粤彩糯2号和粤白甜糯7号的种子由广东省农业科学院作物研究所提供。采用随机区组设计,以清水(CK)为对照,设置了5 mmol/L γ-氨基丁酸(GABA)喷施处理,试验小区面积24 m2,3次重复。分别于孕穗前期(9月26日)和孕穗后期(10月2日)于植株下部土面根际土壤喷施药剂和清水。为防药剂的相互交叉污染,喷施时各小区采用泡沫板分隔,
1.2 田间管理
试验的土壤为沙壤土,耕作层的有机质、全氮、全磷、全钾、碱解氮、速效钾和有效磷分别为8.6 g/kg、0.526 g/kg、108 mg/kg、62 mg/kg、80 mg/kg、52 mg/kg和39 mg/kg,土壤的pH值为6.3。试验于2020年8月22日播种,8月29日间苗和定苗。种植前,采用中拖耙耕地起畦,畦宽为1.4 m,每畦种植2行玉米,种植密度48 000株/hm2。
玉米种子直播时同步基肥施用基肥,施肥量为300 kg/hm2(挪威复合肥(N-P-K=15-15-15))。苗期、小喇叭口期配合小培土和大喇叭口期配合大培土均追施挪威复合肥(21-6-13),各时期的肥料施用量均为300 kg/hm2。
播种前应用XDSY-1 种衣剂(药种质量比1∶50)对种子进行处理,同时应用木霉菌颗粒剂(75 kg/hm2)进行沟施(穴施),以防治苗期和成株期相关病虫为害。于玉米大喇叭口期施用0.01% 芸苔素内酯和阿米西达(嘧菌酯,25% 悬浮剂),防治玉米南方锈病、小斑病和大斑病。
1.3 测定项目及方法
试验全程记载观察田间玉米生育时期、植株农艺性状,于成熟期进行鲜苞产量(kg/hm2)、净穗产量(kg/hm2)、苞叶产量(kg/hm2)等项目调查,计算鲜苞穗叶比,同时对单穗性状如单穗粗(cm)、秃顶长(cm)、穗长(cm)、单穗行数、单穗行粒数和单穗总粒数等开展调查。
分别于灌浆期(抽穗期后12 d)和成熟收获期(抽穗期后26 d)采用LI6400便携式光合测定仪对各处理的糯玉米的叶片光合特性指标进行测定,测定项目主要包括了胞间CO2浓度(Ci)、气孔导度(Gs)、净光合速率(Pn)和蒸腾速率(Tr)等参数。同时,分别于灌浆期和成熟期测定各处理糯玉米穗位上部叶片、穗位叶和穗位下部叶片的平均叶绿素含量SPAD(采用SPAD-502 便携式测定仪),并采用如下公式计算功能叶叶片老化指数:
叶片老化指数=
![]() ×100
×100
分别于抽穗期后12 d(灌浆期)和26 d(成熟收获期)取代表性植株的叶片,测定糯玉米品种叶片的过氧化氢酶(CAT)活性、过氧化物酶(POD)活性和超氧化物歧化酶(SOD)活性以及丙二醛(MDA)含量[12,16]。
采用Microsoft Excel 2010 对试验数据进行录入和整理计算,采用SPSS 17.0对试验数据进行方差分析,并采用 Duncan 氏新复极差法(SSR)进行多重比较。
2 结果与分析
2.1 孕穗期根施GABA对糯玉米重要生育时期及农艺性状的影响
孕穗期根施GABA(RS)处理下,供试糯玉米品种的抽穗期、散粉期和叶丝期提前了1~2 d;京科糯928(V2)的株高较对照(CK)显著降低了5.36%,粤白甜糯7号(V3)的株高较CK显著增加了4.61%。RS处理对穗位高度影响不显著(表1)。
表1 孕穗期根施GABA对糯玉米重要生育时期和农艺性状的影响
Tab.1 Effects of GABA root application at booting stage on important growth periods and agronomic characters of waxy corn
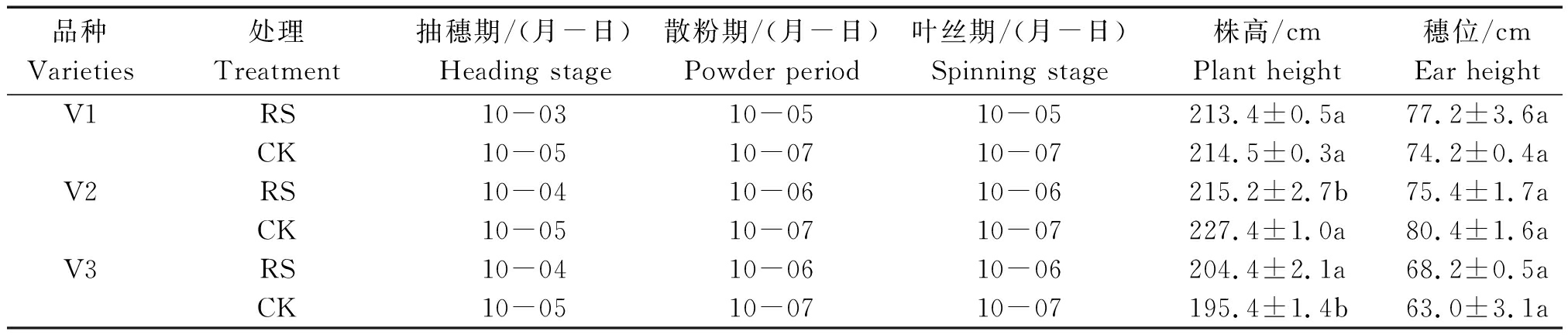
品种Varieties处理Treatment抽穗期/(月-日)Heading stage散粉期/(月-日)Powder period叶丝期/(月-日)Spinning stage株高/cmPlant height穗位/cmEar heightV1RS10-0310-0510-05213.4±0.5a77.2±3.6aCK10-0510-0710-07214.5±0.3a74.2±0.4aV2RS10-0410-0610-06215.2±2.7b75.4±1.7aCK10-0510-0710-07227.4±1.0a80.4±1.6aV3RS10-0410-0610-06204.4±2.1a68.2±0.5aCK10-0510-0710-07195.4±1.4b63.0±3.1a
注:同一品种不同处理间的小写字母表示差异显著P<0.05。表2同。
Note:Lowercase letters between different treatments of the same variety represents significant difference at P<0.05. The same as Tab.2.
2.2 根施GABA对糯玉米光合特性的影响
与CK相比,RS处理V1灌浆期的净光合速率显著提高了13.90%,胞间二氧化碳浓度显著降低了11.69%。RS处理对灌浆期的气孔导度影响不显著。相对于CK,RS处理显著提高了灌浆期V1和V3的叶片蒸腾速率(图1)。
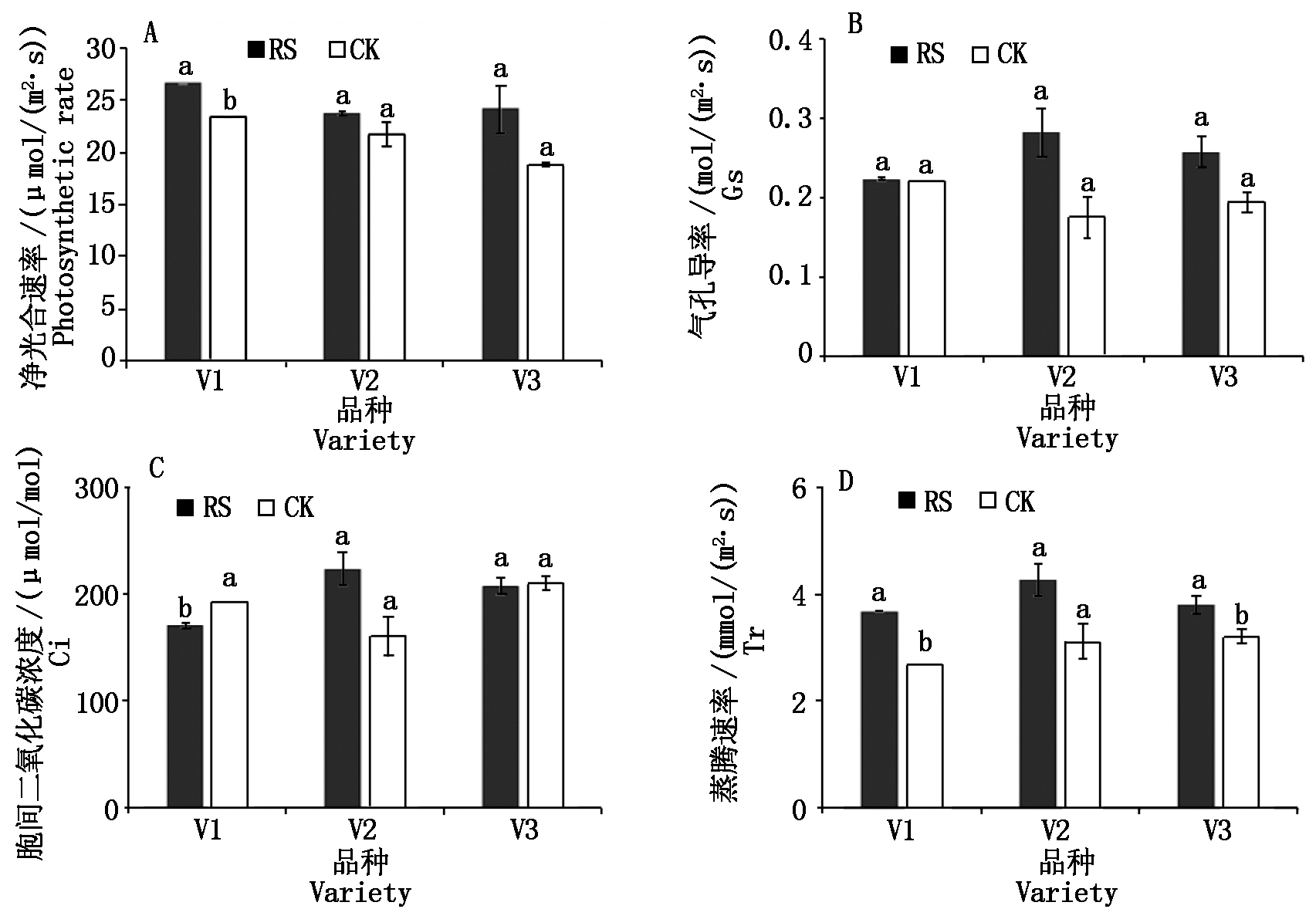
图1 孕穗期根施GABA对糯玉米灌浆期叶片光合参数的影响
Fig.1 Effect of GABA application at booting stage on photosynthetic parameters of waxy corn at filling stage
与CK相比,RS处理显著提高了糯玉米品种V1、V2和V3灌浆期叶片的净光合速率,分别提高了10.85%,18.44%,2.64%。RS处理显著提高了糯玉米品种V1灌浆期叶片的气孔导度和蒸腾速率。相对于CK,RS处理降低了灌浆期V3的叶片胞间二氧化碳浓度(图2)。
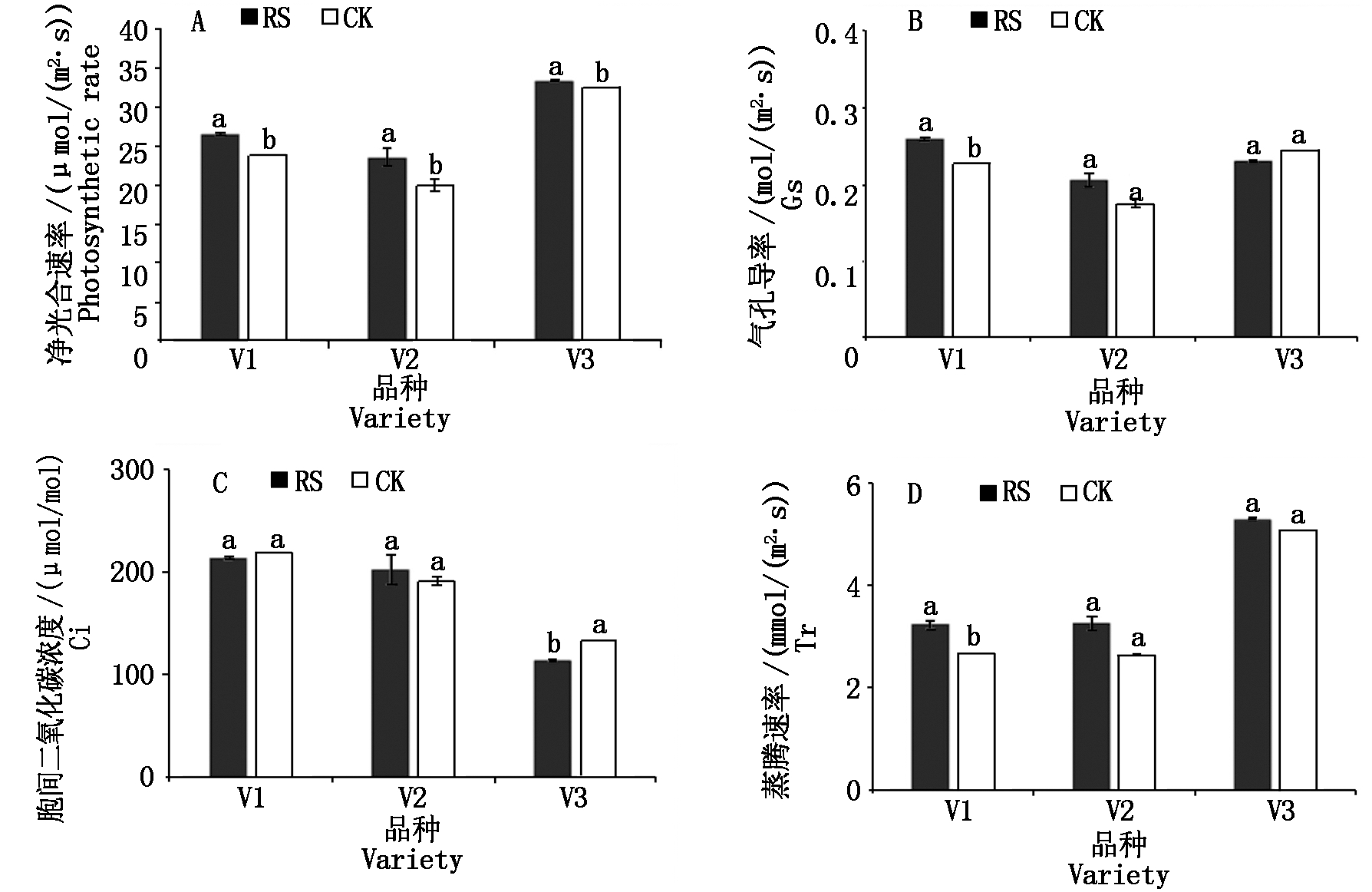
图2 孕穗期根施GABA对糯玉米成熟期叶片光合参数的影响
Fig.2 Effect of GABA application at booting stage on photosynthetic parameters in leaves of waxy corn at mature stage
2.3 根施GABA对糯玉米功能叶叶绿含量及衰老特性的影响
与CK相比,RS处理对供试品种灌浆期各部位叶片的SPAD和叶片老化指数的影响差异不显著(图3)。
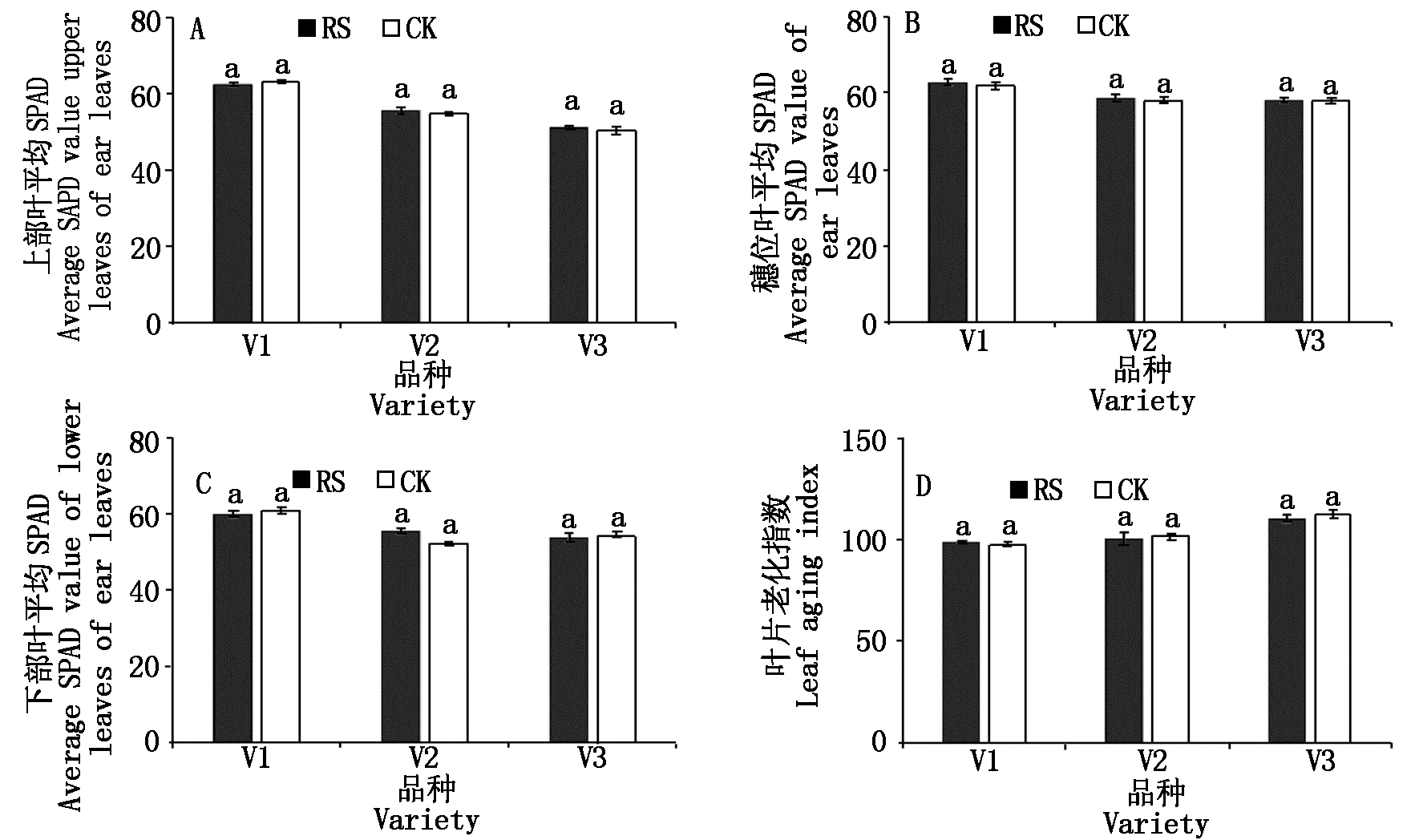
图3 孕穗期根施GABA对糯玉米灌浆期功能叶SPAD值和叶片老化指数的影响
Fig.3 Effect of GABA root application at booting stage on SPAD value and leaf
aging index of functional leaves of waxy corn at filling stage
与CK相比,RS处理显著降低了V2成熟期上部叶的SPAD,对穗位叶SPAD影响差异不显著。相对于CK,RS处理V1和V2成熟期下部叶的SPAD分别显著提高了11.01%和16.08%。RS处理下V1成熟期的叶片老化指数显著提高(图4)。
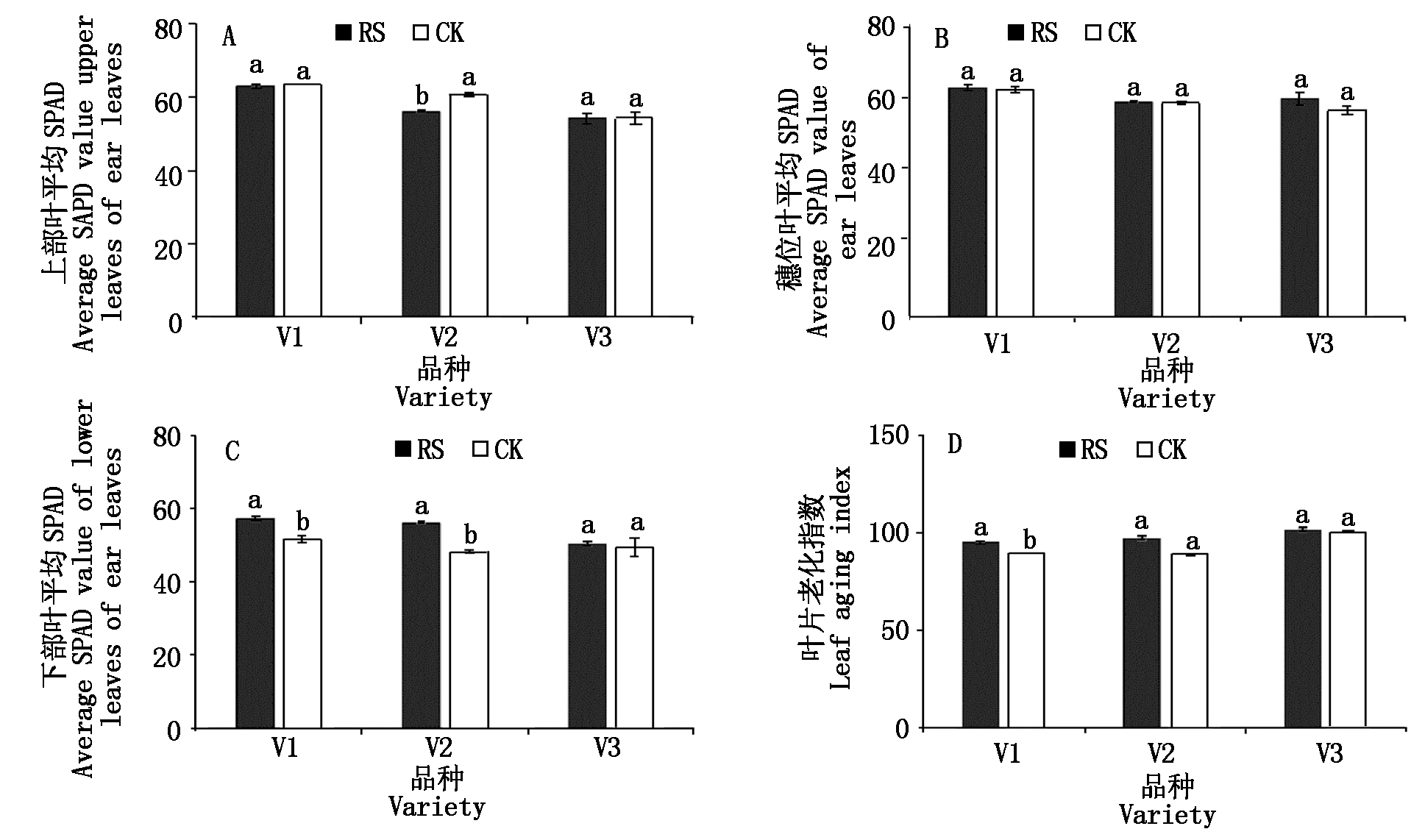
图4 孕穗期根施GABA对糯玉米成熟期功能叶SPAD值和叶片老化指数的影响
Fig.4 Effect of GABA root application at booting stage on SPAD value and
leaf aging index of functional leaves of waxy corn at mature stage
2.4 根施GABA对糯玉米抗氧化生理的影响
与CK相比,RS处理对灌浆期功能叶的SOD和POD活性(以鲜质量计)无显著影响,糯玉米品种V1叶片的CAT活性(以鲜质量计)在RS处理下显著提高,糯玉米品种V3叶片的CAT活性和MDA含量(以鲜质量计)在RS处理下显著降低(图5)。
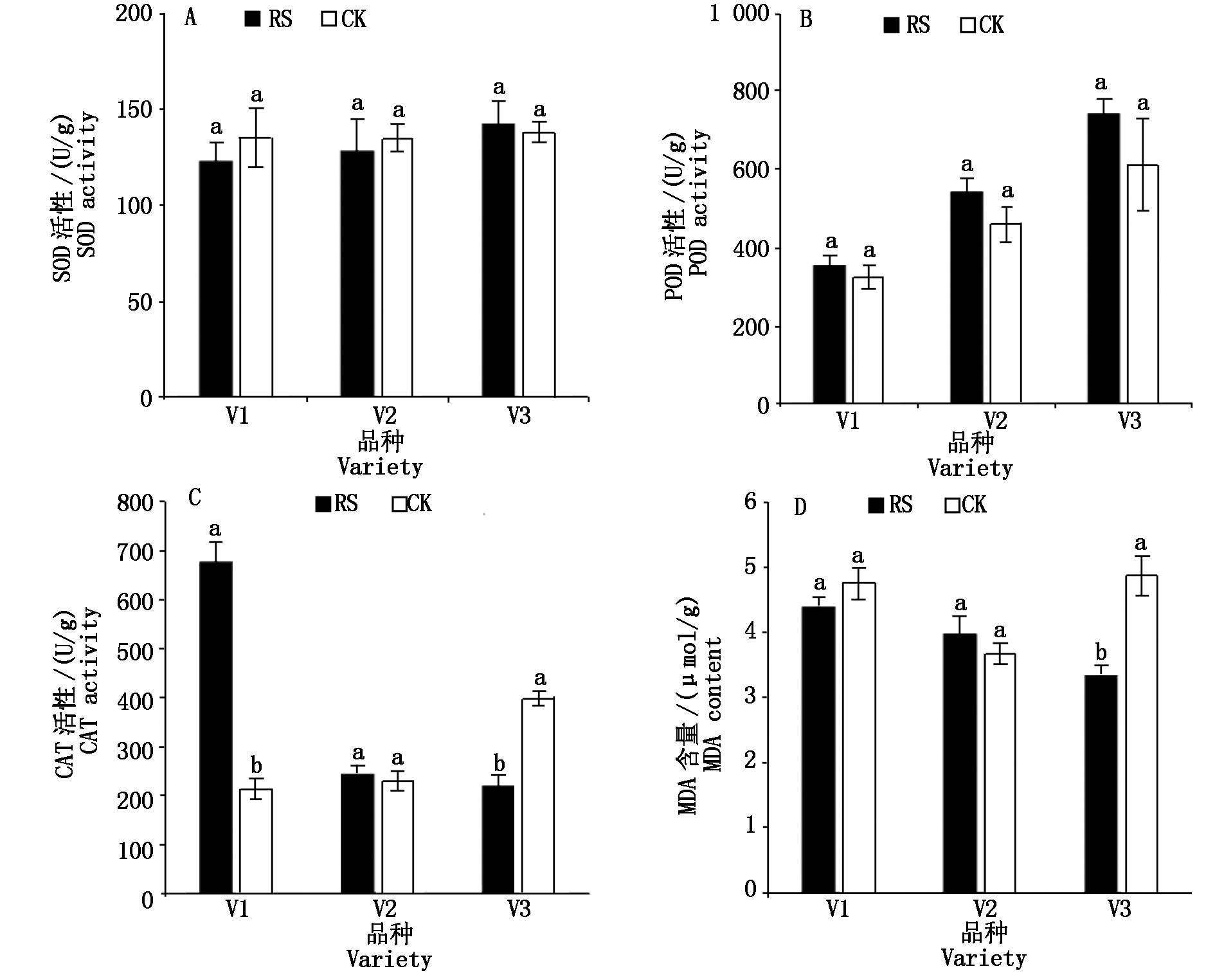
图5 孕穗期根施GABA对糯玉米灌浆期功能叶叶片抗氧化生理的影响
Fig.5 Effect of root application of GABA on antioxidant physiology in functional leaves of waxy corn at filling stage
与CK相比,RS处理对成熟期功能叶的SOD活性无显著影响,显著降低了V2和V3的功能叶POD活性,显著提高了供试品种的功能叶CAT活性,显著降低了供试品种功能叶的MDA含量(图6)。
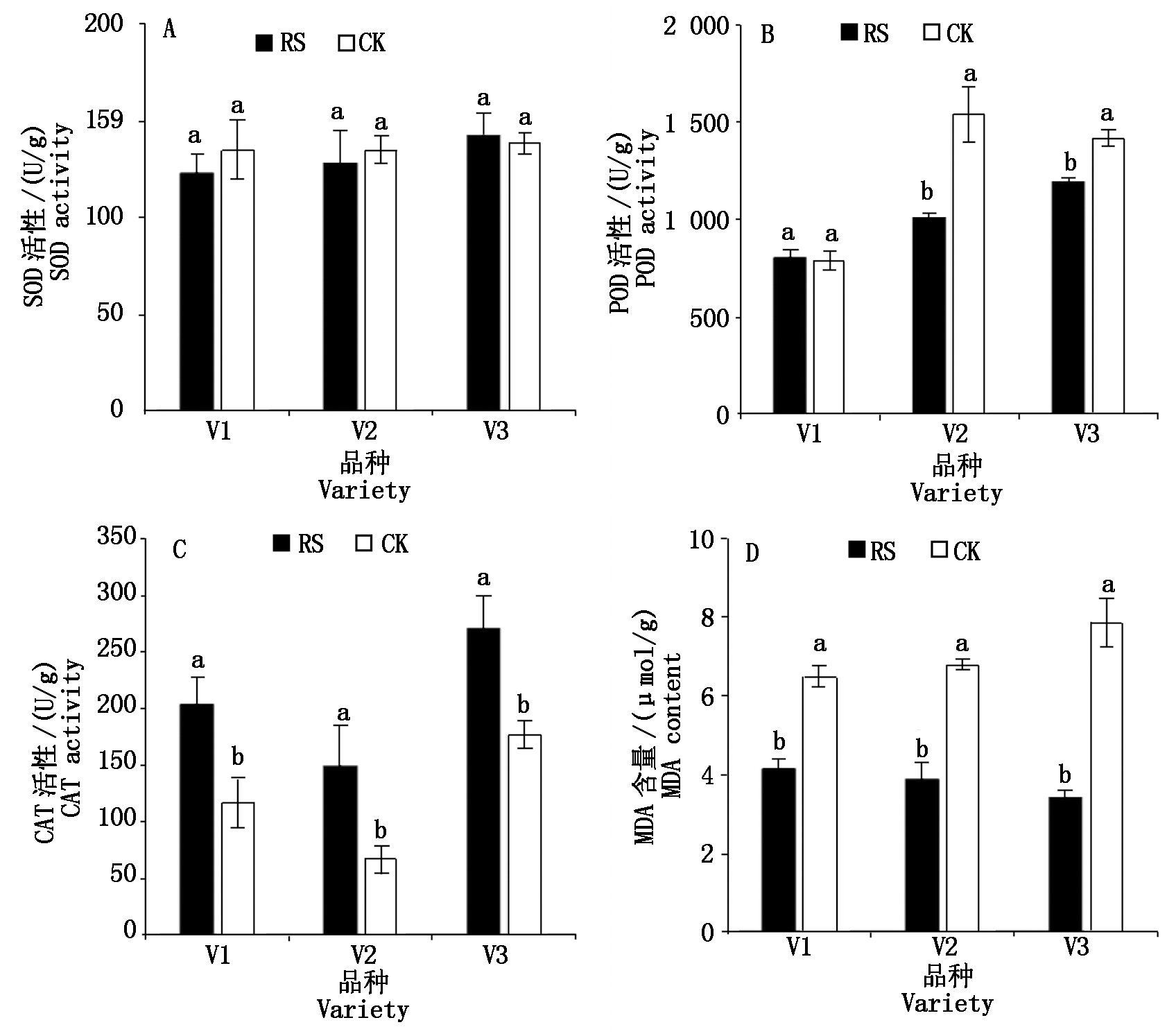
图6 孕穗期根施GABA对糯玉米成熟期功能叶叶片抗氧化生理的影响
Fig.6 Effect of root application of GABA on antioxidant physiology in functional leaves of waxy corn at mature stage
2.5 根施GABA对糯玉米产量构成因子的影响
与CK相比,RS显著提高了V2的穗长和V1的总粒数,分别提高了5.26%,12.23%。RS处理对糯玉米的秃顶长、穗粗、穗行数和行粒数等指标影响差异不显著(表2)。
表2 孕穗期根施GABA对糯玉米产量构成的影响
Tab.2 Effect of GABA root application at booting stage on yield components of waxy corn
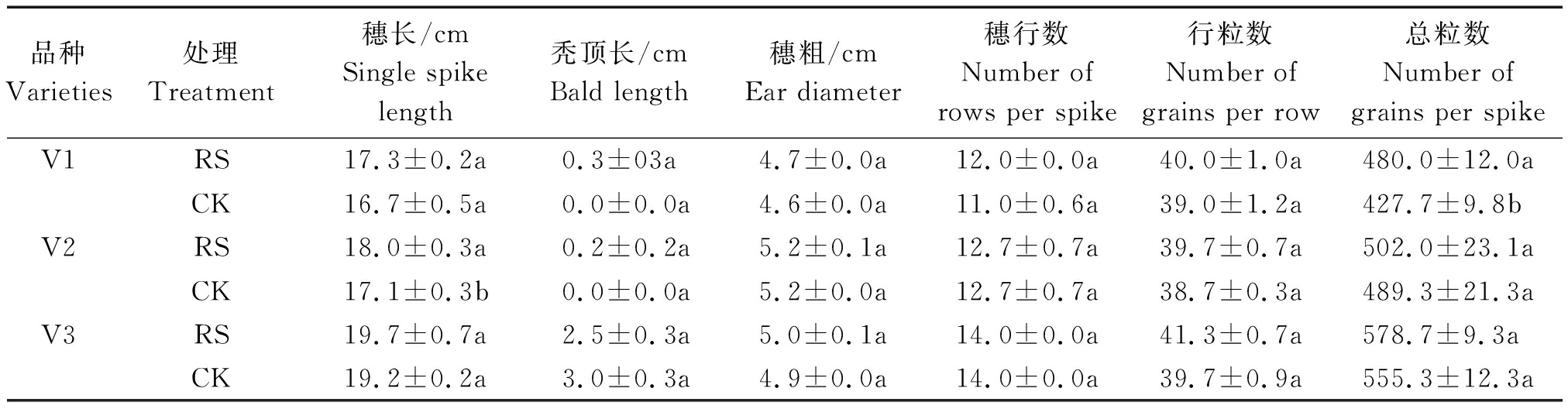
品种Varieties处理Treatment穗长/cmSingle spike length秃顶长/cmBald length穗粗/cmEar diameter穗行数Number of rows per spike行粒数Number of grains per row总粒数Number ofgrains per spikeV1RS17.3±0.2a0.3±03a4.7±0.0a12.0±0.0a40.0±1.0a480.0±12.0aCK16.7±0.5a0.0±0.0a4.6±0.0a11.0±0.6a39.0±1.2a427.7±9.8bV2RS18.0±0.3a0.2±0.2a5.2±0.1a12.7±0.7a39.7±0.7a502.0±23.1aCK17.1±0.3b0.0±0.0a5.2±0.0a12.7±0.7a38.7±0.3a489.3±21.3aV3RS19.7±0.7a2.5±0.3a5.0±0.1a14.0±0.0a41.3±0.7a578.7±9.3aCK19.2±0.2a3.0±0.3a4.9±0.0a14.0±0.0a39.7±0.9a555.3±12.3a
2.6 根施GABA对糯玉米产量的影响
与对照相比,RS处理提高了供试糯玉米品种的净穗产量,降低了供试糯玉米品种的苞叶产量,其中V2的净穗产量提高和苞叶产量减低均达显著水平;RS处理显著降低了V1的鲜苞产量,对其余2个品种的鲜苞产量影响不显著;RS处理显著提高了V2的鲜苞穗叶比值(图7)。
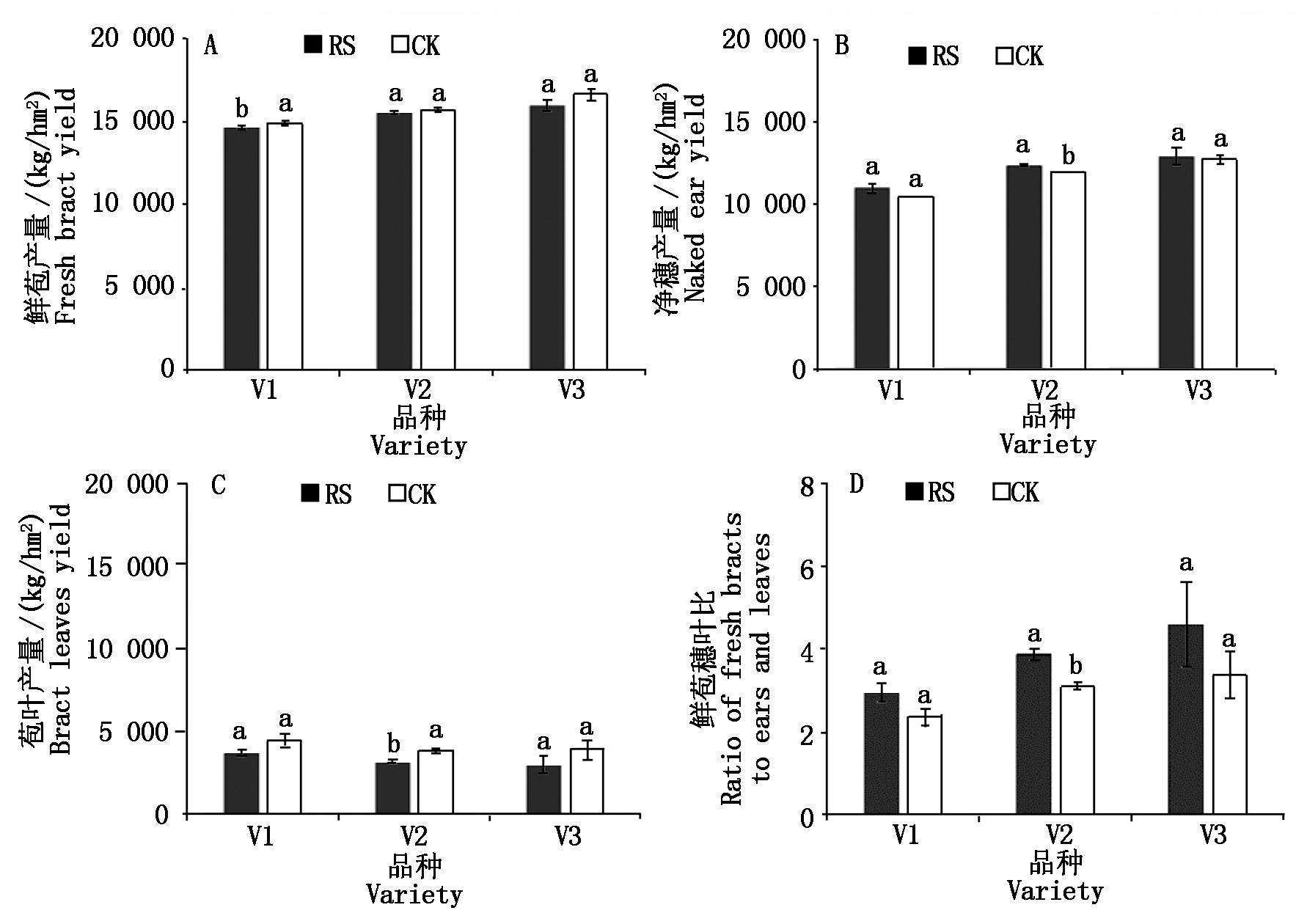
图7 孕穗期根施GABA对糯玉米产量的影响
Fig.7 Effect of root application of GABA on waxy corn yield
2.7 相关性分析
相关性分析表明,净穗产量与鲜苞产量、鲜苞穗叶比、穗长、穗行数、总粒数和灌浆期叶片老化指数等指标呈显著正相关关系(P<0.05)。净穗产量与灌浆期穗位上部叶平均SPAD值、成熟期穗位上部叶平均SPAD值、灌浆期穗位叶平均SPAD值、成熟期穗位叶平均SPAD值、灌浆期穗位下部叶平均SPAD值和成熟期叶片胞间CO2浓度呈显著负相关关系(P<0.05)(图8)。
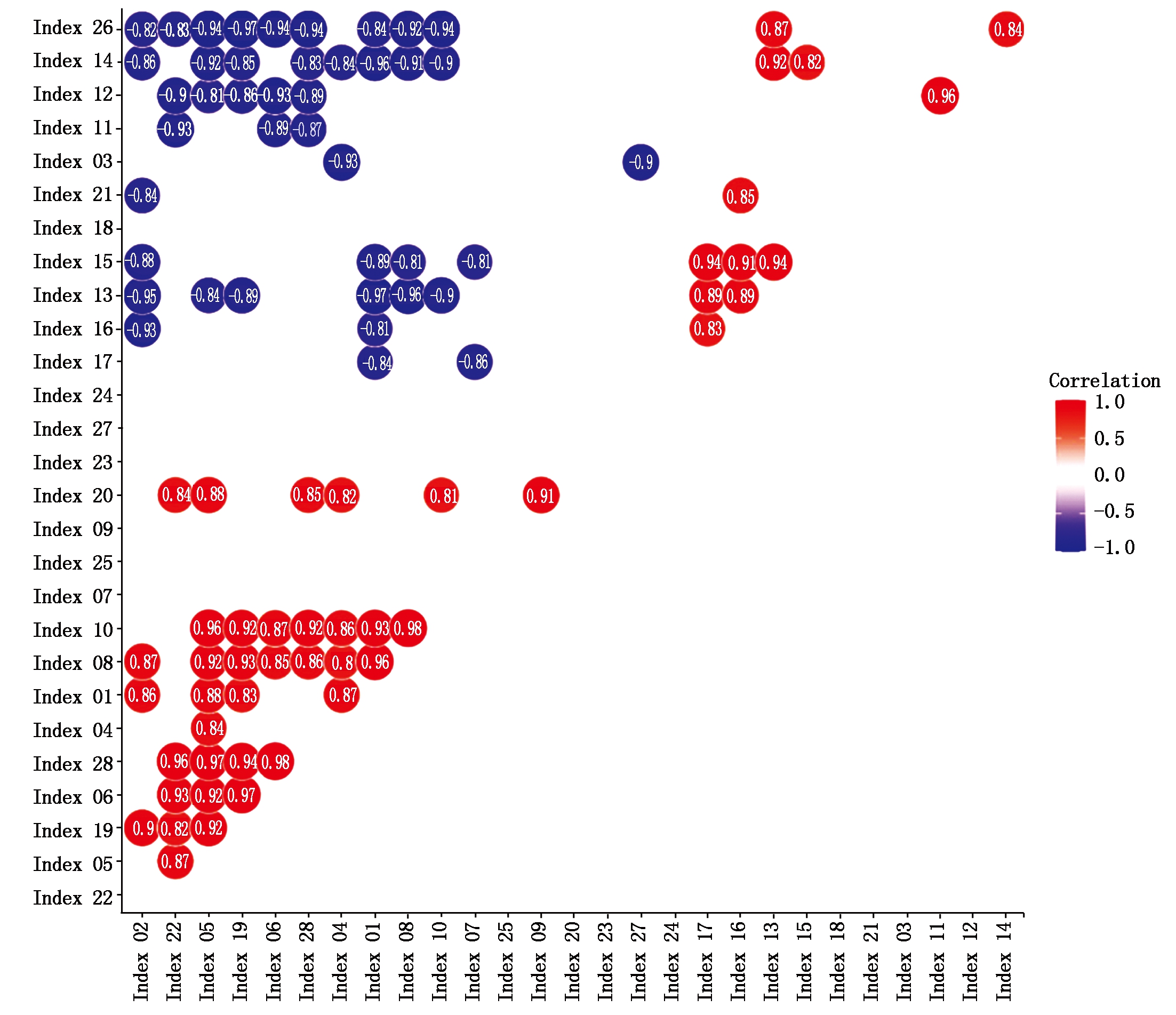
Index 01.净穗产量;Index 02.鲜苞产量;Index 03.苞叶产量;Index 04.鲜苞穗叶比;Index 05.穗长;Index 06.秃顶长;Index 07.穗粗;Index 08.穗行数;Index 09.行粒数;Index 10.总粒数;Index 11.株高;Index 12.穗位;Index 13.灌浆期穗位上部叶平均SPAD值;Index 14.成熟期穗位上部叶平均SPAD值;Index 15.灌浆期穗位叶平均SPAD值;Index 16.成熟期穗位叶平均SPAD值;Index 17.灌浆期穗位下部叶平均SPAD值;Index 18.成熟期期穗位下部叶平均SPAD值;Index 19.灌浆期叶片老化指数;Index 20.成熟期期叶片老化指数;Index 21.灌浆期净光合速率;Index 22.灌浆期净光合速率;Index 23.灌浆期叶片气孔导度;Index 24.成熟期期叶片气孔导度;Index 25.灌浆期叶片胞间CO2浓度;Index 26.成熟期叶片胞间CO2浓度;Index 27.灌浆期叶片蒸腾速率;Index 28.成熟期叶片蒸腾速率。
Index 01.Naked ear yield;Index 02.Fresh bract yield;Index 03.Bract leaves yield;Index 04.Ratio of fresh bracts to ears and leaves;Index 05.Single spike length;Index 06.Bald length;Index 07.Ear diameter;Index 08.Number of rows per spike;Index 09.Number of grains per row;Index 10.Number of grains per spike;Index 11.Plant height;Index 12.Ear height;Index 13.Average SPAD value of upper leaves of ear leaves at filling stage;Index 14.Average SPAD value of upper leaves of ear leaves at mature stage;Index 15.Average SPAD value of ear leaves at filling stage;Index 16.Average SPAD value of ear leaves at mature stage;Index 17.Average SPAD value of lower leaves of ear leaves;Index 18.Average SPAD value of lower leaves of ear leaves;Index 19.Leaf aging index at filling stage;Index 20.Leaf aging index at filling stage;Index 21. Photosynthetic rate of leaf at filling stage;Index 22.Photosynthetic rate of leaf at filling stage;Index 23.Conductance to H2O at filling stage;Index 24.Conductance to H2O of leaf at filling stage;Index 25.Intercellular CO2 concentration of leaf at filling stage;Index 26.Intercellular CO2 concentration of leaf at filling stage;Index 27.Transpiration rate of leaf at filling stage;Index 28.Transpiration rate of leaf at filling stage.
图8 测定指标的相关性分析
Fig.8 Correlation analysis of the investigated parameters
3 讨论与结论
γ-氨基丁酸(GABA)是一种新发现的信号分子,参与植物在最佳和胁迫环境下的生理过程、生长和发育[17]。GABA参与了多种生理代谢机制,导致玉米形态生长的改善[16]。逆境胁迫下,外源施用GABA可以促进玉米植株的内源GABA积累,同时提高抗氧化酶活性,影响内源激素水平,以达到了保护根系免受氧化损伤的效果,促进了根系活力的提高,最终使得根系的生长良好,影响根系吸收和运转营养物质[18-20];外源GABA还可影响植物通气组织的形成,促进了逆境条件下玉米幼苗生长,并能维持玉米植株在响应逆境过程中根系结构[21]。施加外源GABA,还能改善玉米植株营养生长参数[22]。有证据显示,可以通过外源施用抑制剂(GAD或SSADH)影响玉米幼苗的GABA积累,进而影响了玉米幼苗的抗逆境能力;外源喷施GABA能提高玉米幼苗中抗氧化酶(SOD和POD)活性,降低了MDA的积累[23]。有研究指出,GABA为玉米幼苗生长发育提供营养,即在不同浓度的营养液处理下,施用GABA利于玉米幼苗的生长发育,且在低浓度营养液处理下更显著[24]。有研究还发现,GABA可以影响细胞分化,高和低GABA浓度条件下分别表现为抑制和促进植物茎的伸长[25]。本研究结果进一步表明,孕穗期根施5 mmol/L GABA处理下,3个供试糯玉米品种的抽穗期、散粉期和叶丝期均比对照提前了1~2 d,并对成熟期株高有一定影响,且在品种间存在显著差异。
GABA的多方面作用还被认为是通过改善光合功能和调节氧化应激来最小化逆境胁迫对作物的有害影响[26-27]。外源施用GABA可以减少盐胁迫对光合系统的损害,提高光合作用和叶绿素荧光参数[28]。前期研究发现,玉米品种粤白糯6号和正甜68在外源GABA处理后第3天幼苗净光合速率比空白对照分别提高了25.13%,12.94%,而粤白糯6号、正甜68和粤彩糯2号在处理后第7天净光合速率则分别提高了12.45%,11.23%,13.19%;3个玉米品种的蒸腾速率和气孔导度变化趋势相似,粤彩糯2号在外源GABA处理第3天和第7天分别增加了8.93%,32.72%[16]。外源GABA的施用促进了玉米苗期的净光合作用、气体交换能力和叶绿素生物合成,施用GABA可改善玉米幼苗的净光合作用和气体交换,这可能是由于维持了细胞膨压,促进了叶绿素生物合成,通过调节各种生理生化过程减少了氧化损伤。本研究结果进一步证明,孕穗期根施外源GABA一定程度提高了灌浆期和成熟期的净光合速率和蒸腾速率。
GABA调控植物生长发育进程与形态建成、光合作用与物质积累、功能叶衰老快慢等形态生理过程甚至缓解多种逆境胁迫,从而进一步调控产量形成。本研究结果还表明,孕穗期根施GABA提高了糯玉米成熟期叶片老化指数,有效延缓了功能叶衰老。GABA转氨酶在植物发育过程中表现出显著的调节功能,它参与将GABA还原为琥珀酸半醛,并参与GABA分流途径,该途径在植物生命周期的衰老阶段对氮代谢起着至关重要的作用。在各种胁迫条件下,GABA转氨酶基因的功能丧失诱导植物叶片早期衰老[29-31]。此外,有证据显示,通过外源GABA处理诱导了高温胁迫下玉米幼苗的内源GABA积累,提高了光合作用和产量[32]。王晓冬等[33]指出,受涝渍胁迫后的小麦幼苗经适宜浓度的外源GABA处理能提高成熟期小麦干物质积累和产量。郑舒文等[34]也证明外源GABA处理可以缓解渍水对小麦的胁迫,提高小麦产量。杨娜等[35]研究指出,喷施100 mg/L GABA处理提高了小麦的穗粒数和产量。沙汉景等[36]的研究指出,单一的外源喷施GABA、Pro和SA或三者的复混喷施能缓解水稻的盐胁迫危害,提高水稻的产量,其中盐敏感品种受到的有利调控最显著。谷海涛[37]研究认为,干旱胁迫处理下喷施GABA能够促进寒地粳稻产量形成、氮代谢和提高内源GABA含量,不同品种孕穗期干旱胁迫下喷施外源GABA的最适浓度有差异。本研究结果表明,RS处理显著提高了成熟期功能叶CAT活性,显著降低了功能叶的MDA含量。总的来说,与CK相比,孕穗期根施GABA处理提高了供试糯玉米品种的净穗产量(1.62%~4.97%),降低了供试糯玉米品种的苞叶产量(16.52%~23.09%),其中粤白甜糯7号的净穗产量提高和苞叶产量减低均达显著水平;净穗产量与鲜苞产量、鲜苞穗叶比、穗长、穗行数、总粒数和灌浆期叶片老化指数呈显著正相关关系(P<0.05)。因此,孕穗期根施GABA处理可以通过调控糯玉米品种的光合生理、抗逆生理、产量构成因子和鲜苞的苞叶占比等影响糯玉米品种的净穗产量提高。
总的来说,孕穗期根施GABA处理通过影响糯玉米品种的光合生理、抗逆生理、产量构成因子和鲜苞的苞叶占比等来调控糯玉米品种的净穗产量。
致谢:感谢莫钊文博士对本研究提出的建议;助理研究员聂俊、江炳志,本科生蔡若琪、黄一凤、郑燕虹,参加了部分试验观测和数据分析工作,特此致谢!
[1] 陈传晓,董志强,高娇,徐田军,焦浏,卢霖,张凤路. 聚糠萘水剂对不同积温带春玉米灌浆期光合性能的影响[J].玉米科学,2013,21(3):66-70,75. doi:10.13597/j.cnki.maize.science.2013.03.015.
Chen C X,Dong Z Q,Gao J,Xu T J,Jiao L,Lu L,Zhang F L. Effects of PASP-KT-NAA on the photosynthetic performances of different maize cultivars in different accumulated temperature zones[J].Journal of Maize Sciences,2013,21(3):66-70,75.
[2] 高娇,董志强,徐田军,陈传晓,焦浏,卢霖,董学瑞. 聚糠萘水剂对不同积温带玉米花后叶片氮同化的影响[J].生态学报,2014,34(11):2938-2947. doi:10.5846/stxb201212101773.
Gao J,Dong Z Q,Xu T J,Chen C X,Jiao L,Lu L,Dong X R. Effects of PASP-KT-NAA on maize leaf nitrogen assimilation after florescence over different temperature gradients[J].Acta Ecologica Sinica,2014,34(11):2938-2947.
[3] 王庆燕,管大海,潘海波,李建民,段留生,张明才,李召虎.油菜素内酯对春玉米灌浆期叶片光合功能与产量的调控效应[J].作物学报,2015,41(10):1557-1563. doi:10.3724/SP.J.1006.2015.01557.
Wang Q Y,Guan D H,Pan H B,Li J M,Duan L S,Zhang M C,Li Z H. Effect of brassinolide on leaf photosynthetic function and yield in spring maize filling stage[J].Acta Agronomica Sinica,2015,41(10):1557-1563.
[4] 卢霖,董志强,董学瑞,焦浏,李光彦,高娇. 乙矮合剂对不同密度夏玉米花粒期叶片氮素同化与早衰的影响[J].作物学报,2015,41(12):1870-1879. doi:10.3724/SP.J.1006.2015.01870.
Lu L,Dong Z Q,Dong X R,Jiao L,Li G Y,Gao J. Effects of ethylene-chlormequat-potassium on leaf nitrogen assimilation after anthesis and early senescence under different planting densities[J].Acta Agronomica Sinica,2015,41(12):1870-1879.
[5] 叶德练,王玉斌,周琳,李建民,段留生,张明才,李召虎. 乙烯利和氮肥对夏玉米氮素吸收与利用及产量的调控效应[J].作物学报,2015,41(11):1701-1710. doi:10.3724/SP.J.1006.2015.01701.
Ye D L,Wang Y B,Zhou L,Li J M,Duan L S,Zhang M C,Li Z H. Effect of ethephon and nitrogen fertilizer on nitrogen uptake,nitrogen use efficiency and yield of summer maize[J].Acta Agronomica Sinica,2015,41(11):1701-1710.
[6] 李光彦,王庆燕,许艳丽,卢霖,焦浏,董学瑞,董志强. 双重化控对春玉米灌浆期穗位叶和籽粒蔗糖代谢关键酶活性的影响[J].2016,42(8):1215-1223. doi:10.3724/SP.J.1006.2016.01215.
Li G Y,Wang Q Y,Xu Y L,Lu L,Jiao L,Dong X R,Dong Z Q. Effect of plant growth regulators on key enzymes in sucrose metabolism of ear leaf and grain at filling stage of spring maize[J].Acta Agronomica Sinica,2016,42(8):1215-1223.
[7] Fait A,Fromm H,Walter D,Galili G,Fernie A R. Highway or byway:the metabolic role of the GABA shunt in plants[J].Trends in Plant Science,2008,13(1):14-19. doi:10.1016/j.tplants.2007.10.005.
[8] Fait A,Nesi A N,Angelovici R,Lehmann M,Pham P A,Song L H,Haslam R P,Napier J A,Galili G,Fernie A R. Targeted enhancement of glutamate-to-γ-aminobutyrate conversion in Arabidopsis seeds affects carbon-nitrogen balance and storage reserves in a development-dependent manner[J].Plant Physiology,2011,157(3):1026-1042. doi:10.1104/pp.111.179986.
[9] Renault H,El Amrani A,Berger A,Mouille G,Soubigou-Taconnat L,Bouchereau A,Deleu C. γ-Aminobutyric acid transaminase deficiency impairs central carbon metabolism and leads to cell wall defects during salt stress in Arabidopsis roots[J].Plant,Cell & Environment,2013,36(5):1009-1018. doi:10.1111/pce.12033.
[10] Salvatierra A,Pimentel P,Almada R,Hinrichsen P. Exogenous GABA application transiently improves the tolerance to root hypoxia on a sensitive genotype of Prunus rootstock[J].Environmental and Experimental Botany,2016,125:52-66. doi:10.1016/j.envexpbot.2016.01.009.
[11] Vijayakumari K,Puthur J T. γ-Aminobutyric acid(GABA)priming enhances the osmotic stress tolerance in Piper nigrum Linn. plants subjected to PEG-induced stress[J].Plant Growth Regulation,2016,78(1):57-67. doi:10.1007/s10725-015-0074-6.
[12] Soleimani Aghdam M,Naderi R,Jannatizadeh A,Sarcheshmeh M A A,Babalar M. Enhancement of postharvest chilling tolerance of Anthurium cut flowers by γ-aminobutyric acid(GABA)treatments[J].Scientia Horticulturae,2016,198:52-60. doi:10.1016/j.scienta.2015.11.019.
[13] Roberts M R.Does GABA act as a signal in plants? Hints from molecular studies[J].Plant Signaling & Behavior,2007,2(5):408-409.doi:10.4161/psb.2.5.4335.
[14] Batushansky A,Kirma M,Grillich N,Toubiana D,Pham P A,Balbo I,Fromm H,Galili G,Fernie A R,Fait A. Combined transcriptomics and metabolomics of Arabidopsis thaliana seedlings exposed to exogenous GABA suggest its role in plants is predominantly metabolic[J].Molecular Plant,2014,7(6):1065-1068. doi:10.1093/mp/ssu017.
[15] Mekonnen D W,Flügge U I,Ludewig F. Gamma-aminobutyric acid depletion affects stomata closure and drought tolerance of Arabidopsis thaliana[J].Plant Science,2016,245:25-34. doi:10.1016/j.plantsci.2016.01.005.
[16] Li W G,Liu J H,Ashraf U,Li G K,Li Y L,Lu W J,Gao L,Han F G,Hu J G. Exogenous γ-aminobutyric acid(GABA)application improved early growth,net photosynthesis,and associated physio-biochemical events in maize[J].Front Plant Sci,2016,7:919. doi:10.3389/fpls.2016.00919.
[17] Khanna R R,Jahan B,Iqbal N,Khan N A,AlAjmi M F,Tabish Rehman M,Khan M I R. GABA reverses salt-inhibited photosynthetic and growth responses through its influence on NO-mediated nitrogen-sulfur assimilation and antioxidant system in wheat[J].Journal of Biotechnology,2021,325:73-82. doi:10.1016/j.jbiotec.2020.11.015.
[18] 王泳超,郑博元,顾万荣,李卓,毛俊,郭家萌,邵瑞鑫,杨青华. γ-氨基丁酸对盐胁迫下玉米幼苗根系氧化损伤及内源激素的调控[J].农药学学报,2018,20(5):607-617. doi:10.16801/j.issn.1008-7303.2018.0078.
Wang Y C,Zheng B Y,Gu W R,Li Z,Mao J,Guo J M,Shao R X,Yang Q H. Γ-Aminobutyric acid on oxidative damage and endogenous hormones in maize seedling roots under salt stress[J].Chinese Journal of Pesticide Science,2018,20(5):607-617.
[19] Hu Y B,Chen B D. Arbuscular mycorrhiza induced putrescine degradation into γ-aminobutyric acid,malic acid accumulation,and improvement of nitrogen assimilation in roots of water-stressed maize plants[J].Mycorrhiza,2020,30(2/3):329-339. doi:10.1007/s00572-020-00952-0.
[20] Saiz-Fern ndez I,Lacuesta M,Pérez-López U,Sampedro M C,Barrio R J,De Diego N. Interplay between 1-aminocyclopropane-1-carboxylic acid,γ-aminobutyrate and D-glucose in the regulation of high nitrate-induced root growth inhibition in maize[J].Plant Science,2020,293:110418. doi:10.1016/j.plantsci.2020.110418.
ndez I,Lacuesta M,Pérez-López U,Sampedro M C,Barrio R J,De Diego N. Interplay between 1-aminocyclopropane-1-carboxylic acid,γ-aminobutyrate and D-glucose in the regulation of high nitrate-induced root growth inhibition in maize[J].Plant Science,2020,293:110418. doi:10.1016/j.plantsci.2020.110418.
[21] Akram S.玉米苗期对渍害的响应及外源调节剂的调节机制[D].武汉:华中农业大学,2018.
Akram S. Response of maize seedling to waterlogging and regulation mechanism of exogenous regulators[D].Wuhan:Huazhong Agricultural University,2018.
[22] Seifikalhor M,Aliniaeifard S,Bernard F,Seif M,Latifi M,Hassani B,Didaran F,Bosacchi M,Rezadoost H,Li T. Γ-Aminobutyric acid confers cadmium tolerance in maize plants by concerted regulation of polyamine metabolism and antioxidant defense systems[J].Scientific Reports,2020,10(1):3356. doi:10.1038/s41598-020-59592-1.
[23] 张华永,崔丽娜,董树亭,高荣岐,孙爱清. 热胁迫诱导玉米幼苗γ-氨基丁酸积累的生理作用[J].山东农业科学,2011,43(7):35-37. doi:10.14083/j.issn.1001-4942.2011.07.030.
Zhang H Y,Cui L N,Dong S T,Gao R Q,Sun A Q. Physiological role of hot stress-induced GABA accumulation in maize seedlings[J].Shandong Agricultural Sciences,2011,43(7):35-37.
[24] 李裕芳,朱昌华,甘立军. γ-氨基丁酸和脲素对玉米幼苗生长的影响[J].生物学杂志,2018,35(3):5-9,22. doi:10.3969/j.issn.2095-1736.2018.03.005.
Li Y F,Zhu C H,Gan L J. The effect of exogenous γ-aminobutyric acid and urea on the growth in maize seedling[J].Journal of Biology,2018,35(3):5-9,22.
[25] Kathiresan A,Miranda J,Chinnappa C C,Reid D M. Γ-aminobutyric acid promotes stem elongation in Stellaria longipes:The role of ethylene[J].Plant Growth Regulation,1998,26(2):131-137. doi:10.1023/A:1006107815064.
[26] Kalhor M S,Aliniaeifard S,Seif M,Asayesh E J,Bernard F,Hassani B,Li T. Title:Enhanced salt tolerance and photosynthetic performance:Implication of γ-amino butyric acid application in salt-exposed lettuce(Lactuca sativa L.)plants[J].Plant Physiology and Biochemistry,2018,130:157-172. doi:10.1016/j.plaphy.2018.07.003.
[27] 贾琰,任鹏飞,赵宏伟,邹德堂,王晋,杨亮. 孕穗期冷水胁迫下施用γ-氨基丁酸对寒地粳稻氮光合效率的调控效应[J].东北农业大学学报,2020,51(1):1-12. doi:10.19720/j.cnki.issn.1005-9369.2020.01.001.
Jia Y,Ren P F,Zhao H W,Zou D T,Wang J,Yang L. Effect of γ-aminobutyric acid on nitrogen photosynthetic efficiency in cold-region Japonica rice under cold water stress at booting stage[J].Journal of Northeast Agricultural University,2020,51(1):1-12.
[28] Wang Y C,Gu W R,Meng Y,Xie T L,Li L J,Li J,Wei S. Γ-aminobutyric acid imparts partial protection from salt stress injury to maize seedlings by improving photosynthesis and upregulating osmoprotectants and antioxidants[J].Scientific Reports,2017,7:43609. doi:10.1038/srep43609.
[29] Ansari M I,Chen S C. Biochemical characterization of gamma-aminobutyric acid(GABA):Pyruvate transaminase during rice leaf senescence[J].International Journal of Integrative Biology,2009,6(1):27-32.
[30] Jalil S U,Ahmad I,Ansari M I. Functional loss of GABA transaminase(GABA-T)expressed early leaf senescence under various stress conditions in Arabidopsis thaliana[J].Current Plant Biology,2017,9/10:11-22. doi:10.1016/j.cpb.2017.02.001.
[31] Uzma Jalil S,Khan M I R,Ansari M I. Role of GABA transaminase in the regulation of development and senescence in Arabidopsis thaliana[J].Current Plant Biology,2019,19:100119. doi:10.1016/j.cpb.2019.100119.
[32] 张华永. γ-氨基丁酸(GABA)对玉米幼苗抗逆性的效应、作用机理及调控[D].泰安:山东农业大学,2010.
Zhang H Y.The effect function and control of γ-aminobutyric acid in maize seedling under adversity stress[D].Taian:Shandong Agricultural University,2010.
[33] 王晓冬,解备涛,李建民,段留生. 外源γ-氨基丁酸(GABA)对小麦苗期耐涝性的影响[J].华北农学报,2010,25(1):155-160.doi:10.7668/hbnxb.2010.01.031.
Wang X D,Xie B T,Li J M,Duan L S. Effects of exogenous GABA on waterlogged tolerance in wheat seedlings[J].Acta Agriculturae Boreali-Sinica,2010,25(1):155-160.
[34] 郑舒文,徐其隆,邹华文. γ-氨基丁酸对渍水胁迫下小麦产量的影响[J].湖北农业科学,2016,55(1):31-33. doi:10.14088/j.cnki.issn0439-8114.2016.01.009.
Zheng S W,Xu Q L,Zou H W. Effect of GABA on yield of wheat under waterlogging condition[J].Hubei Agricultural Sciences,2016,55(1):31-33.
[35] 杨娜,伍宏,甘立军,朱昌华. 叶喷γ-氨基丁酸对小麦产量和品质的影响[J].中国粮油学报,2018,33(3):8-12,20. doi:10.3969/j.issn.1003-0174.2018.03.002.
Yang N,Wu H,Gan L J,Zhu C H. Effect of foliar application of γ-aminobutyric acid on yield and quality of wheat[J].Journal of the Chinese Cereals and Oils Association,2018,33(3):8-12,20.
[36] 沙汉景,胡文成,贾琰,王新鹏,田雪飞,于美芳,赵宏伟. 外源水杨酸、脯氨酸和γ-氨基丁酸对盐胁迫下水稻产量的影响[J].作物学报,2017,43(11):1677-1688. doi:10.3724/SP.J.1006.2017.01677.
Sha H J,Hu W C,Jia Y,Wang X P,Tian X F,Yu M F,Zhao H W. Effect of exogenous salicylic acid,proline,and γ-aminobutyric acid on yield of rice under salt stress[J].Acta Agronomica Sinica,2017,43(11):1677-1688.
[37] 谷海涛. 外源γ-氨基丁酸对孕穗期干旱胁迫下寒地粳稻氮代谢及产量的调控效应[D].哈尔滨:东北农业大学,2018.
Gu H T. Effects of exogenous γ-aminobutyric acid on nitrogen metabolism and yield of Japonica rice in cold region under drought stress at booting stage[D].Harbin:Northeast Agricultural University,2018.
