胰岛素样生长因子结合蛋白3基因(Insulin-like growth factor binding proteins 3,IGFBP-3)是IGFBP家族中含量最高,也是血清中IGF的主要结合蛋白,在多种组织中表达[1]。动物出生后,血液循环中75%~90%的IGF-1与IGFBP-3及酸不稳定亚基(Acid labile subunit,ALS)结合形成三聚体,在调控细胞增殖、生长、分化及动物生长发育方面发挥重要作用[2-3]。IGFBP-3还可以通过自分泌和旁分泌形式在多种组织局部发挥作用。研究发现,骨骼肌可能是IGFBP-3产生的另一自分泌组织[4]。IGFBP-3也可以诱导软骨细胞和骨骼成肌细胞的分化[5],通过IGF依赖性或非依赖性机制调控肌细胞生长[6]。此外,血清中IGFBP-3浓度与生长抑制作用直接相关[7],在小鼠体内过表达IGFBP-3可造成产后生长停滞,代谢缓慢等现象[8]。血清中IGFBP-3的浓度与猪平均日增质量呈负相关[9]。由此表明,IGFBP-3可能在家畜生长发育过程中起着负调控的作用。
研究已报道,IGFBP-3的核苷酸变异影响牛的生产性状和屠宰性状,被认为是与牛生产性状相关的分子标记候选基因[10]。Gao等[11]发现IGFBP-3基因exon2和exon3 C/T多态性与肉牛的体质量、体尺等显著相关;党瑞华等[12]的研究表明,鲁西牛和晋南牛IGFBP-3基因多态与部分屠宰性状有一定关联。此外,IGFBP-3基因的核苷酸变异也影响山羊的屠宰性状。闵令江等[13]对波尔山羊及其杂交后代的研究发现IGFBP-3基因A/G多态与断奶重和眼肌面积有关。这些结果表明,IGFBP-3基因与动物的生长发育及屠宰性能紧密相关。
绵羊(Ovis aries)的IGFBP-3基因位于第4号染色体,包括5个外显子,编码293个氨基酸。目前,IGFBP-3基因在绵羊羊毛性状的关联分析[14]及多态性的分析[15-16]有较少研究,发现IGFBP-3也影响着羊毛的纤维直径。在新西兰9个绵羊群体和藏绵羊IGFBP-3的exon 2-intron 3中并没有发现多态性[17],而与屠宰性状的关联性分析未见报道。
甘肃高山细毛羊是1981年培育而成的毛肉兼用型细毛羊培育品种,对当地高海拔(2 600 m以上)地区具有很好的适应性。然而,也存在着产肉率低、屠宰性能差的缺点[18]。近年来,通过分子遗传育种提高其产肉量及屠宰性能对当地经济具有重要的意义。因此,本试验通过对IGFBP-3基因exon 4-intron 4序列多态性的检测与分析,探讨遗传变异对屠宰性状的影响,为今后甘肃高山细毛羊分子育种提供标记辅助选择基础数据。
1 材料和方法
1.1 试验材料
试验羊只饲养于甘肃省天祝藏族自治县。在羔羊4月龄时颈静脉采集血样592份,每份10 mL,柠檬酸葡萄糖(Acid citrate dextrose,ACD)抗凝,-20 ℃冻存。采用常规的苯酚-氯仿抽提法提取基因组DNA,溶于TE缓冲液,-20 ℃保存。并按公母对等原则屠宰跟踪有生长记录的6月龄羔羊112只,对其宰前活质量、热胴体质量2个胴体性状进行测定;对其中40只羊的肉骨比、眼肌面积、GR值和胴体净肉率等胴体性状及嫩度、pH值、失水率和熟肉率等肉质性状进行测定。
1.2 引物设计和PCR扩增
参考NCBI数据库的绵羊IGFBP-3序列(EU419644.1)设计引物,引物序列为F:5′-GGTTTCTGCTGGTGTGTGG-3′;R:5′-GAGCTCCGTCTTGTGTGTAGG-3′,预期的扩增长度为249 bp。引物由宝生物(大连)工程有限公司合成。PCR反应总体系为25 μL,其中10×缓冲液3.0 μL(含15 mmol/L Mg2+),10 mmol/L上下游引物各0.6 μL,2.5 mmol/L dNTPs 2.0 μL,2.0 U/μL Taq DNA酶 0.4 μL,DNA 模板0.8 μL,添加ddH2O至25 μL。扩增条件:预变性95 ℃ 5 min;变性94 ℃ 30 s,退火58 ℃ 30 s,72 ℃延伸30 s,35个循环;72 ℃延伸10 min,最后4 ℃保存。
1.3 SSCP检测及序列测定
取PCR产物2 μL和变性剂8 μL(0.025%溴酚蓝、98%去离子甲酰胺、10 mmol/L EDTA 和0.025%二甲苯青)充分混匀,98 ℃变性10 min后,立刻冰浴8 min。变性的PCR产物在20 ℃下,14%非变性聚丙烯酰胺凝胶中220 V电泳13 h,待电泳结束后银染显色[19]。经判型分析后,对初步判断的不同基因型纯合子个体的PCR扩增产物用TaKaRa DNA回收试剂盒回收纯化,之后送由华大基因科技有限公司测序。
1.4 统计分析
利用PopGene 32软件计算遗传多态性指标,并进行Hardy-Weinberg平衡检验;采用SPSS 22.0软件进行显著性检验和多重比较。单基因效应方差分析模型如下:P=μ+基因型+随机残差效应,式中P为表型观察值,μ为群体平均值。
2 结果与分析
2.1 IGFBP-3基因exon 4-intron 4的PCR扩增结果
对甘肃高山细毛羊IGFBP-3基因exon 4-intron 4区进行扩增,将扩增产物在1.5%琼脂糖凝胶中进行电泳(图1)。由图可以看出,扩增片段与目的片段大小基本一致且特异性好,可用于下一步SSCP分析。
图1 PCR扩增产物检测
Fig.1 Detection of PCR amplification products
2.2 SSCP检测及序列分析
SSCP分析发现IGFBP-3基因exon 4-intron 4具有出多态性,有3种基因型,分别定义为AA、BB和AB(图2)。通过与GenBank中绵羊IGFBP-3(GenBank EU419644.1)序列比对发现,第4外显子87 bp处发生了A→C颠换(同义突变),intron 4区209处发生C→T转换。
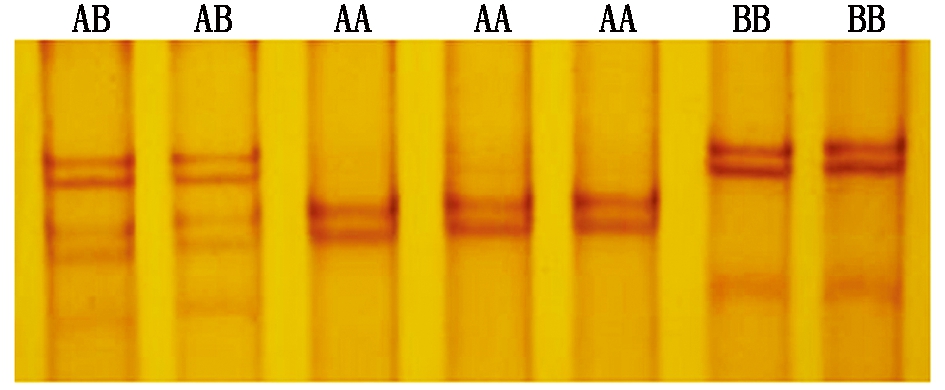
图2 绵羊IGFBP-3基因exon 4-intron 4 SSCP检测
Fig.2 Electrophoresis patterns of exon 4-intron 4 of
IGFBP-3 SSCP analysis
2.3 IGFBP-3基因多态位点的遗传分析
结果表明,A和B 2个等位基因的等位基因频率分别为56.76%,43.24%,AA、AB和BB 3种基因型的基因型频率分别为29.73%,54.05%,16.22%(表1)。AB基因型是优势基因型,A等位基因为优势等位基因。exon 4-intron 4呈中度多态,卡方检验表明该位点处于Hardy-Weinberg平衡状态。
表1 甘肃高山细毛羊IGFBP-3第4外显子区的遗传多态性分析
Tab.1 Genetic diversity of of IGFBP-3 exon 4 in Gansu alpine merino
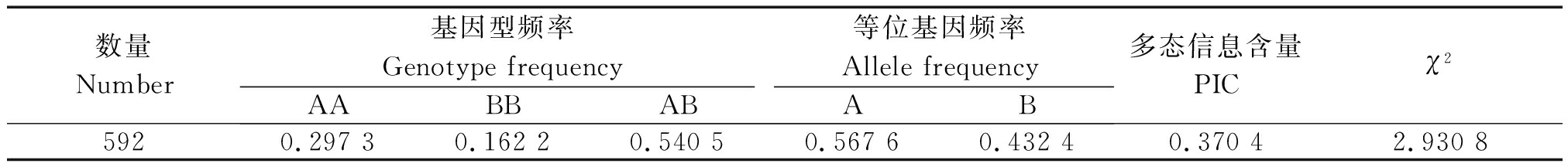
数量Number基因型频率Genotype frequency等位基因频率Allele frequencyAABBABAB多态信息含量PICχ25920.297 30.162 20.540 50.567 60.432 4 0.370 42.930 8
2.4 IGFBP-3基因多态性与屠宰性状的相关性分析
对甘肃高山细毛羊IGFBP-3基因第4外显子的3种不同基因型和屠宰性状进行了相关性分析。结果表明:基因型与大部分性状存在相关性(表2)。由表2可以看出:3种基因型个体的宰前活质量的最小二乘均值BB型和AB型都高于AA型(P<0.01),BB型大于AB型,但差异不显著(P>0.05);热胴体质量BB型极显著高于AB型和AA型(P<0.01);而AB型高于AA型,但无显著差异(P>0.05);眼肌面积和GR值BB型均显著高于AB型和AA型(P<0.05);嫩度值BB型显著高于AA型(P<0.05)。
表2 IGFBP-3基因exon 4-intron 4区域基因型对胴体和肉质性状的影响
Tab.2 Effects of different genotypes at exon 4-intron 4 of IGFBP-3 on carcass and meat quality traits
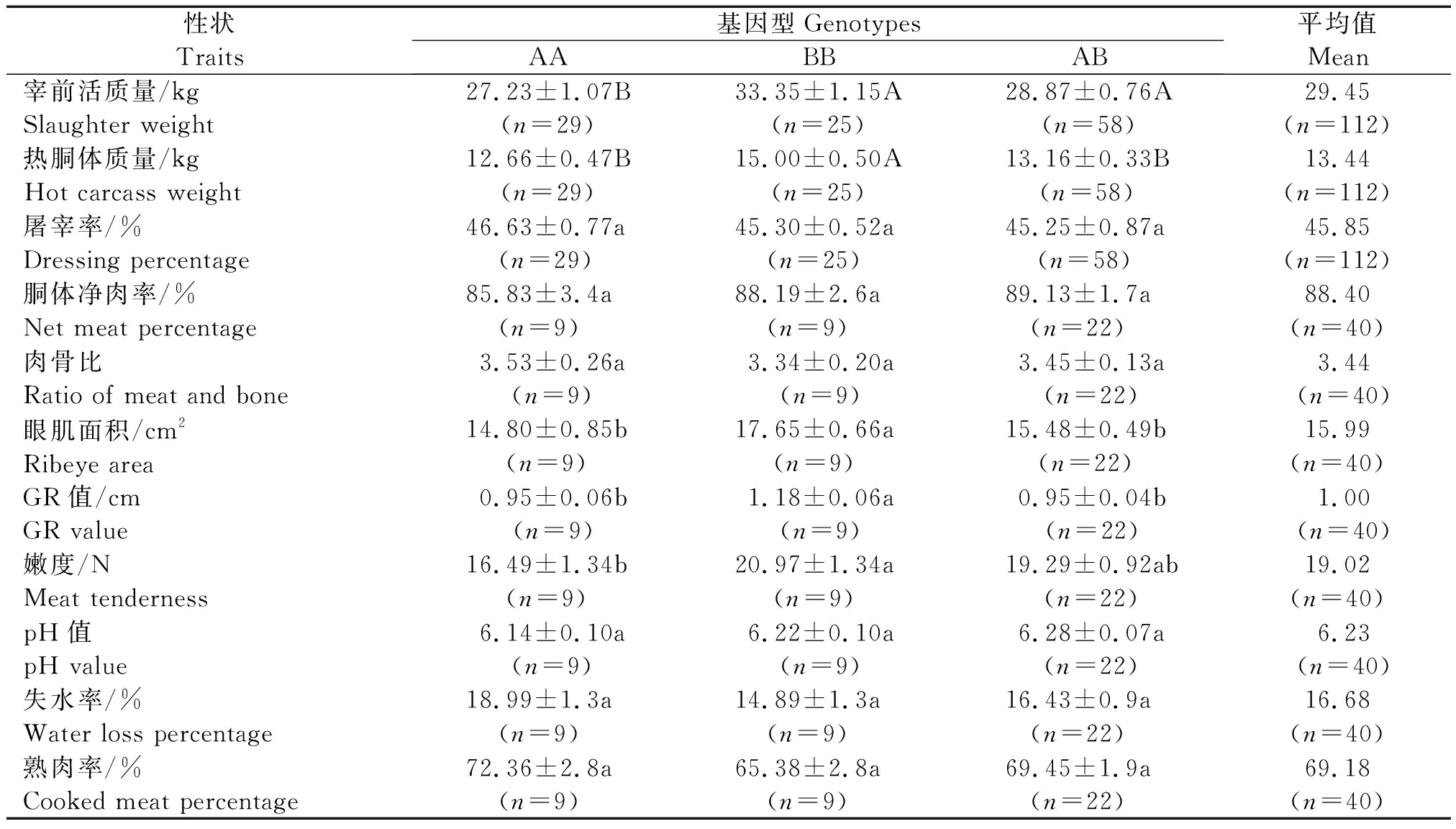
性状Traits基因型GenotypesAABBAB平均值Mean宰前活质量/kgSlaughter weight 27.23±1.07B(n=29)33.35±1.15A(n=25)28.87±0.76A(n=58)29.45(n=112)热胴体质量/kgHot carcass weight12.66±0.47B(n=29)15.00±0.50A(n=25)13.16±0.33B(n=58)13.44(n=112)屠宰率/%Dressing percentage46.63±0.77a(n=29)45.30±0.52a(n=25)45.25±0.87a(n=58)45.85(n=112)胴体净肉率/%Net meat percentage85.83±3.4a(n=9)88.19±2.6a(n=9)89.13±1.7a(n=22)88.40(n=40)肉骨比Ratio of meat and bone3.53±0.26a(n=9)3.34±0.20a(n=9)3.45±0.13a(n=22)3.44(n=40)眼肌面积/cm2Ribeye area 14.80±0.85b(n=9)17.65±0.66a(n=9)15.48±0.49b(n=22)15.99(n=40)GR值/cmGR value0.95±0.06b(n=9)1.18±0.06a(n=9)0.95±0.04b(n=22)1.00(n=40)嫩度/NMeat tenderness 16.49±1.34b(n=9)20.97±1.34a(n=9)19.29±0.92ab(n=22)19.02(n=40)pH值pH value6.14±0.10a(n=9)6.22±0.10a(n=9)6.28±0.07a(n=22)6.23(n=40)失水率/%Water loss percentage 18.99±1.3a(n=9)14.89±1.3a(n=9)16.43±0.9a(n=22)16.68(n=40)熟肉率/%Cooked meat percentage72.36±2.8a(n=9)65.38±2.8a(n=9)69.45±1.9a(n=22)69.18(n=40)
注:数值用平均值±标准误表示。同行相同字母间表示差异不显著(P>0.05),不同小写字母间表示差异显著(P<0.05),不同大写字母间表示差异极显著(P<0.01)。括号内数字为个体数。
Note:Date in the table showed as means s.Values in each line with same letters mean insignificant difference (P>0.05);Different lowercase letters mean significant difference (P<0.05);Different capital letter mean extremely distinct difference (P<0.01).Numbers in the parentheses indicate the number of lambs.
3 讨论
本试验以IGFBP-3基因作为调控绵羊屠宰性状的候选基因,通过PCR-SSCP方法对该基因exon 4-intron 4区域进行单核苷酸多态性检测,发现在外显子4中存在一处碱基颠换:A87C,内含子4区存在一处C209T的转换。基因型分析结果表明,以杂合型AB个体最多,BB型个体最少,但χ2适合性检验表明该群体处于Hardy-Weinberg平衡状态,这或许与甘肃高山细毛羊长期处于相对闭塞的祁连山区,自交繁育,在长期的生产实践中所受的选择强度小有关。
对3种基因型与屠宰性状的最小二乘分析显示:甘肃高山细毛羊IGFBP-3基因对宰前活质量、热胴体质量有极显著影响;对眼肌面积、GR值、嫩度有显著影响。表现为BB型对应性状显著或极显著大于AA型,且呈现出BB>AB>AA的趋势。与本研究结果相似,在猪[20]、牛[11]、山羊[21]等群体中IGFBP-3基因的突变也影响其屠宰性状和生产性状。例如,宋玉芹等[20]研究发现,IGFBP-3第2内含子A307G多态位点与猪的生长性状有关,其GG基因型猪具有体高、瘦肉率高和生长较快的优点。Gao等[11]的研究表明,IGFBP-3第2外显子的核苷酸变异与牛的体质量和体尺等显著相关,BB基因型牛的胸围显著高于AA型的,24,36月龄尻宽显著高于AA型,BB基因型是优势基因型。孙维斌等[22]的研究表明,秦川牛AB和BB基因型个体胴体脂肪含量高于AA型个体,但AA型个体的眼肌面积大于BB型个体。李美玉等[21]对山羊IGFBP-3基因与经济性状的关系分析时发现,该基因多态与3月龄体长和胸围有极显著影响,且与断奶体质量和眼肌面积有关,但AG基因型均显著或极显著大于GC型和GG型,推测以超显性为主。同样,有研究表明,IGFBP-3基因与猪的背膘厚和肉色[23-24],眼肌面积和胴体瘦肉率等均有关系[25]。
在本试验中,IGFBP-3基因第4外显子区存在3种基因型,A为优势等位基因,AB为优势基因型,且BB基因型个体的屠宰性状优于AB型和AA型个体。这与目前甘肃高山细毛羊的生产实际相符,甘肃高山细毛羊为毛肉兼用细毛羊,产肉性能一般。肉主毛从是甘肃高山细毛羊的选育方向。近年来,羊肉的消费需求越来越大,不断提高B等位基因在群体中的频率,选择BB型个体进行纯繁,有利于提高甘肃高山细毛羊的屠宰性能。
[1] Hjortebjerg R,Frystyk J.Determination of IGFs and their binding proteins[J].Best Pract Res Clin Endocrinol Metab,2013,27(6):771-781.doi:10.1016/j.beem.2013.08.010.
[2] Wu Q Y,Yu H,Fang X B,Cheng Y Y,Dong L J,Wei W Z,Wang G,Fu H Y,Liu S C,Hao L L.The association of haplotypes in IGFBP-3 gene promoter region and tissue expressions in three pig breeds[J]. Animal Cells and Systems,2016,20(6):384-393.doi:10.1080/19768354.2016.1253614.
[3] Superchi P,Saleri R,Borghetti P,Ferrarini G,Cavalli V,Sereni M,Zavattini S,Sabbioni A.Effects of a dietary crude fibre concentrate on growth in weaned piglets[J].Animal,2017,11(11):1905-1912.doi:10.1017/S175173111700057X.
[4] Foulstone E J,Savage P B,Crown A L,Holly J M P,Stewart C E H.Role of insulin-like growth factor binding protein-3 (IGFBP-3) in the differentiation of primary human adult skeletal myoblasts[J].J Cell Physiol,2003,195(1):70-79.doi:10.1002/jcp.10227.
[5] O'Rear L,Longobardi L,Torello M,Law B K,Moses H L,Chiarelli F,Spagnoli A.Signaling cross-talk between IGF-binding protein-3 and transforming growth factor-(beta) in mesenchymal chondroprogenitor cell growth[J].J Mol Endocrinol,2005,34(3):723-737.doi:10.1677/jme.1.01746.
[6] Jones J I,Clemmons D R.Insulin-like growth factors and their binding proteins:biological actions[J].Endocr Rev,1995,16(1):3-34.doi:10.1210/edrv-16-1-3.
[7] Ohde D,Walz M,Walz C,Noce A,Brenmoehl J,Langhammer M,Hoeflich A.Sex-specific control of muscle mass:elevated IGFBP proteolysis and reductions of IGF-1 levels are associated with substantial loss of carcass weight in male DU6PxIGFBP-2 transgenic mice[J].Cells,2020,9(10):2174.doi:10.3390/cells9102174.
[8] Modric T,Silha J V,Shi Z D,Gui Y T,Suwanichkul A,Durham S K,Powell D R,Murphy L J.Phenotypic manifestations of insulin-like growth factor-binding protein-3 overexpression in transgenic mice[J].Endocrinology,2001,142(5):1958-1967.doi:10.1210/endo.142.5.8165.
[9] Clutter A C,Spicer L J,Woltmann M D,Grimes R W,Hammond J M,Buchanan D S.Plasma growth hormone,insulin-like growth factor I,and insulin-like growth factor binding proteins in pigs with divergent genetic merit for postweaning average daily gain[J].J Anim Sci,1995,73(6):1776-1783.doi:10.2527/1995.7361776x.
[10] Choudhary V,Kumar P,Bhattacharya T K,Bhushan B,Sharma A,Shukla A.DNA polymorphism of insulin-like growth factor-binding protein-3 gene and its association with birth weight and body weight in cattle[J].J Anim Breed Genet,2007,124(1):29-34.doi:10.1111/j.1439-0388.2007.00626.x.
[11] Gao X,Shi M Y,Xu X R,Li J Y,Ren H Y,Xu S Z.Sequence variations in the bovine IGF-I and IGFBP3 genes and their association with growth and development traits in Chinese beef cattle [J].Journal of Integrative Agriculture,2009,8(6):717-722.doi:10.1016/S1671-2927(08)60270-9.
[12] 党瑞华,魏伍川,陈宏,蓝贤勇,胡沈荣,苏利红.IGFBP3基因多态性与鲁西牛和晋南牛部分屠宰性状的相关性[J].中国农学通报,2005,21(3):19-22.doi:10.3969/j.issn.1000-6850.2005.03.006.
Dang R H,Wei W C,Chen H,Lan X Y,Hu S R,Su L H.Polymorphisms of insulin-like growth factor binding protein 3 gene and its associations with several carcass traits in Luxi and Jinnan cattle[J].Chinese Agricultural Science Bulletin,2005,3:19-22.
[13] 闵令江,沈伟,李美玉,刘焕奇,陈宏,潘庆杰.山羊IGFBP-3基因多态性与体质量、屠体性状的关系[J].广西农业生物科学,2006,25(S):56-58.
Min L J,Shen W,Li M Y,Liu H Q,Chen H,Pan Q J.Relationship between IGFBP-3 gene polymorphism and body weight traits and carcass traits in goat[J].Journal of Guangxi Agricultural and Biological Science,2006,25(S):56-58.
[14] 沈敏,王文君,杨永林,甘尚权,马春萍,何其宏,张永胜,李宁,刘守仁.利用PCR-SacⅡ-RFLP技术检测绵羊IGFBP-3基因多态性的研究[J].新疆农业科学,2010,47(5):849-853.
Shen M,Wang W J,Yang Y L,Gan S Q,Ma C P,He Q H,Zhang Y S,Li N,Liu S R.PCR-SacⅡ-RFLP detecting polymorphism at the sheep IGFBP-3 gene[J].Xinjiang Agricultural Sciences,2010,47(5):849-853.
[15] 于姣,陈宏,蓝贤勇,潘传英,孙维斌,张润锋,房兴堂,刘波.5种家畜IGFBP3基因遗传变异研究[J].西北农林科技大学学报(自然科学版),2007,35(3):33-37.doi:10.3321/j.issn:1671-9387.2007.03.008.
Yu J,Chen H,Lan X Y,Pan C Y,Sun W B,Zhang R F,Fang X T,Liu B.Genetic diversity of IGFBP3 gene among five domestic animal species[J].Journal of Northwest Sci-Tech University of Agriculture and Forestry (Natural Science Edition),2007,35(3):33 -37.
[16] 沈敏,王文君,杨永林,甘尚权,何其宏,张永胜,王建华,马春萍,刘正山,刘守仁,李宁.IGFBP-3基因多态性及其与中国美利奴羊部分羊毛性状的关联性分析[J].遗传,2008,30(9):1182-1186.doi:10.3321/j.issn:0253-9772.2008.09.014.
Shen M,Wang W J,Yang Y L,Gan S Q,He Q H,Zhang Y S,Wang J H,Ma C P,Liu Z S,Liu S R,Li N.A novel polymorphism of IGFBP-3 gene and its relationship with several wool traits in Chinese Merino sheep[J].Heredity,2008,30(9):1182-1186.
[17] 闫伟.绵羊FABP4、IGFBP3和SPP1基因遗传变异对脂肪,生长和胴体性状的影响[D].兰州:甘肃农业大学,2013.
Yan W.Effect of FABP4, IGFBP3 and SPP1 variations on fat,growth and carcass traits in sheep[D].Lanzhou:Gansu Agricultural University,2013.
[18] 赵有璋.羊生产学[M].3版.北京:中国农业出版社,2011.
Zhao Y Z.Sheep production science[M].The 3rd edition.Beijing:China Agriculture Press,2011.
[19] Byun S O,Fang Q,Zhou H,Hickford J G H.An effective method for silver-staining DNA in large numbers of polyacrylamide gels[J].Anal Biochem,2009 ,385(1):174-175.doi:10.1016/j.ab.2008.10.024.
[20] 宋玉芹,于光辉,王建琳,张灿,张廷荣.IGFBP3基因内含子2多态性及其与猪主要生长性状的关联分析[J].中国畜牧杂志,2017,53(7):44-48.doi:10.19556/j.0258-7033.2017-07-044.
Song Y Q,Yu G H,Wang J L,Zhang C,Zhang T R.The polymorphism of IGFBP3 intron 2 and its relationship with main growth traits of pigs [J].Chinese Journal of Animal Science,2017,53(7):44-48.
[21] 李美玉,李兰,闵令江,王建民.山羊IGFBP-3基因的遗传分析及其与经济性状的关系[J].畜牧兽医学报,2008,39(12):1647-1653.doi:10.3321/j.issn:0366-6964.2008.12.004.
Li M Y,Li L,Min L J,Wang J M.Genetic analysis of IGFBP-3 gene and its association with economic traits in gost[J].Acta Veterinaria et Zootechnica Sinica,2008,39(12):1647-1653.
[22] 孙维斌,陈宏,雷雪芹,雷初朝,张英汉,李瑞彪,昝林森,胡沈荣. IGFBP3基因多态性与秦川牛部分屠宰性状的相关性[J].遗传,2003,25(5):511-516.doi:10.3321/j.issn:0253-9772.2003.05.003.
Sun W B,Chen H,Lei X Q,Lei C H,Zhang Y J,Li R B,Zan L S,Hu S R.Polymorphisms of insulin-like growth factor binding protein 3 gene and its associations with several carcass traits in Qinchuan cattle[J].Inheritance,2003,25(5):511-516.
[23] Saito T,Akutsu S,Urushiyama T,Ishibashi K,Nakagawa Y,Shuler C F,Yamane A.Changes in the mRNA expressions of insulin-like growth factors,their receptors,and binding proteins during the postnatal development of rat masseter muscle[J].Zoolog Sci,2003,20(4):441-447.doi:10.2108/zsj.20.441.
[24] Wang W J,Meng Q Y,Hu X X,Li N.Genetic variation and association of insulin-like growth factor binding protein-3 with performance in swine[J].Biochem Genet,2009,47(3/4):315-321.doi:10.1007/s10528-009-9230-x.
[25] Liu D W,Zhang H,Wu Z F,Li J Q,Yang G F,Zhang X Q.Identification of SNPs and their effects on swine growth and carcass traits for porcine IGFBP-3 gene[J].Agricultural Sciences in China,2008,7(5):630-635.doi:10.1016/S1671-2927(08)60062-0.
