在玉米生产中,高秆品种易倒伏,通常造成严重的减产;而矮秆玉米经济系数高,抗倒伏能力强,光能利用率高,利于密植高产。目前,已被定位的矮秆单基因有60多个(http://www.maizegdb.org/),包括隐性单基因br1、br2、d1、d2和d3等,显性单基因D8、D9、D11和Dt等,其中br2[1]、d3[2]、D8[3]、D9[4]和Dt[5]等基因已被克隆;分布于10条染色体上,已定位的控制玉米株高的QTL位点300余个(http://www.maizegdb.org/),但目前玉米中能够被应用的矮秆资源有限,遗传多样性低,主要集中于br2[6]。因此,为发掘新的玉米矮秆种质资源,并了解其遗传特性,对推动玉米矮化育种具有重要意义。本研究中,以自然突变所获得的矮秆突变体K718d为主要材料,研究其农艺经济性状表现和对外源激素敏感性;通过遗传交配设计,分析该矮秆性状的遗传模式,并初步定位该矮秆基因,为之后基因的精细定位、克隆及育种中的利用提供参考依据。
1 材料和方法
1.1 供试材料
野生型K718;矮秆突变体K718d(P1);5个高秆测验自交系(P2):K1208、Q3、Ly118、Na2和K338;以及K718d与测验自交系组配的正反交F1、BC1、BC2、F2等群体。5个已知矮秆基因材料114F(br2)、110K(br1)、301(cr1)、K15d(br2突变体)、K123d(br2突变体)与K718d杂交配制的F1组合。所有材料均由四川正红生物技术有限责任公司(简称公司)提供。
1.2 试验设计
1.2.1 K718d与K718形态差异比较 于2019年在公司双流区育种基地种植K718d与K718,选取其中30个有代表性的单株,对株高、穗位高等主要农艺性状进行考察;各小区中收获有代表性的10个果穗,考察主要经济性状。用t测验检测性状差异显著性,并对植株和果穗拍照对比。
1.2.2 K718d激素敏感性研究 选取K718d与K718种子浸种催芽,播于发芽盒,随机区组设计,3次重复,5个浓度处理,每处理25株,于两叶一心期用0,25,50,75,100 μg/mL的GA3和IAA溶液隔1 d浇灌1次,20 d后测量苗高及第一叶鞘长度;内源激素GA3测定参照徐敏等[7]方法测定。测定结果进行方差分析和多重比较分析(Excel 2010,Spss 20.0)。
1.2.3 K718d矮秆性状遗传模式分析 试验于四川崇州和雅安两生态地进行。各种植正反交F1群体84粒,BC1、BC2群体各168粒,F2群体各420粒。进行分离群体的株高鉴定,χ2检验株高分离比例,并确定遗传模式。
1.2.4 K718d矮化基因定位 定位群体为K718d×Ly118 F2,于四川崇州育种基地播种2 184粒。单株编号挂牌,调查记录高、矮植株。采用BSA-SSR法[8]进行基因定位。从定位群体中选取极高、极矮株各20株,提取DNA后等量混合,用于构建高、矮秆基因池。选取458对SSR引物于亲本K718d和Ly118间和高、矮基因池间筛选出多态性引物后,在定位群体中的所有矮秆单株间进行扩增。DNA提取用2×CTAB法,PCR体系为25 μL,用3%琼脂糖凝胶对扩增产物进行电泳检测,采用MAPMAKER 3.0、MAPDraw V2.1[9]软件进行连锁分析及遗传图谱的绘制。
1.2.5 K718d矮秆基因等位性鉴定 K718d与5个已知矮秆基因材料组配F1,并种植于公司海南陵水基地,各组合种植28粒,成熟期间观察F1株高表现,确定基因等位关系。
2 结果与分析
2.1 K718d与K718主要性状比较
K718与K718d形态对比见图1。K718d植株和穗位矮化,基部节间缩短,节间数减少,果穗变短。K718d与K718果穗均结实较好,筒型、籽粒半马齿型,但K718籽粒略长,K718d籽粒略宽。
K718d与K718主要性状差异列于表1。与K718相比,K718d生育期显著延长,株高、穗位高、节间数和节间长度分别减少48.23%,75.57%,30.83%和65.92%,差异均达极显著水平;K718d穗粗增加8.31%,穗行数和百粒质量差异不显著,但穗长和行粒数分别极显著降低28.57%,28.47%,导致单株产量极显著降低36.44%。
表1 K718d与K718主要农艺和经济性状比较
Tab.1 Comparisons of the main agronomic and economic traits between the K718d and K718
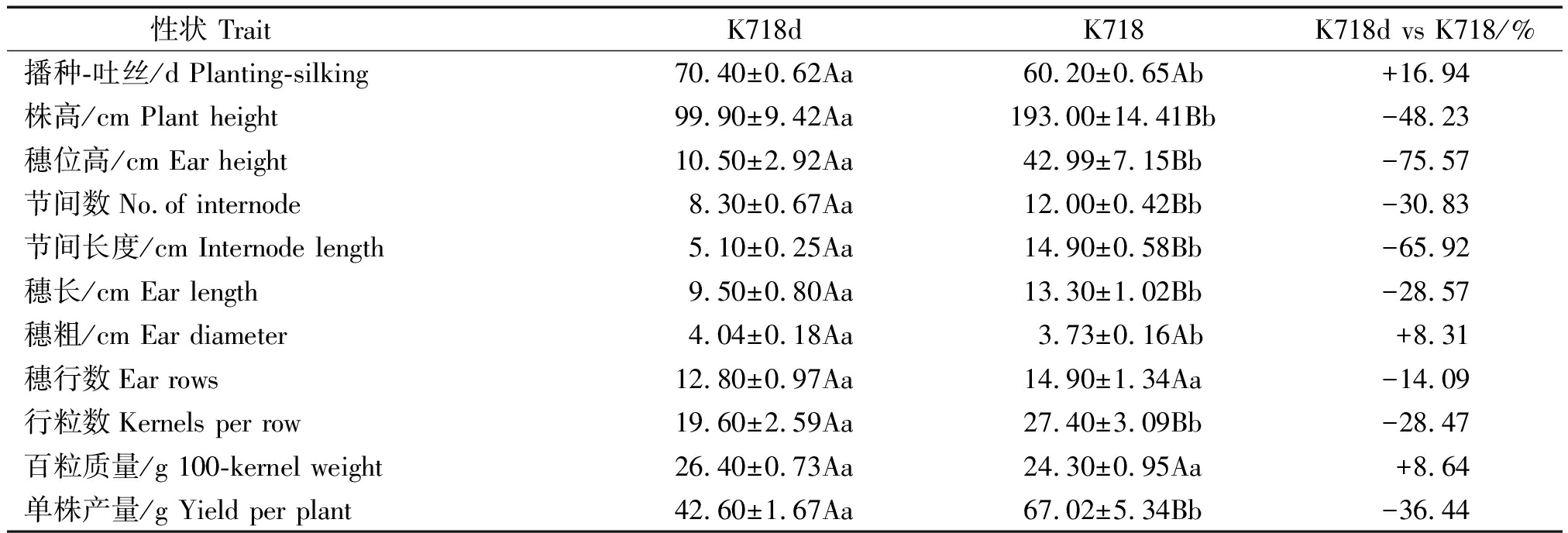
性状 Trait K718dK718K718d vs K718/%播种-吐丝/d Planting-silking70.40±0.62Aa60.20±0.65Ab+16.94株高/cm Plant height99.90±9.42Aa193.00±14.41Bb-48.23穗位高/cm Ear height10.50±2.92Aa42.99±7.15Bb-75.57节间数No.of internode8.30±0.67Aa12.00±0.42Bb-30.83节间长度/cm Internode length5.10±0.25Aa14.90±0.58Bb-65.92穗长/cm Ear length9.50±0.80Aa13.30±1.02Bb-28.57穗粗/cm Ear diameter4.04±0.18Aa3.73±0.16Ab+8.31穗行数Ear rows12.80±0.97Aa14.90±1.34Aa-14.09行粒数Kernels per row19.60±2.59Aa27.40±3.09Bb-28.47百粒质量/g 100-kernel weight26.40±0.73Aa24.30±0.95Aa+8.64单株产量/g Yield per plant42.60±1.67Aa67.02±5.34Bb-36.44
注:不同小写字母表示在P=0.05水平差异显著;不同大写字母表示在P=0.01水平差异显著。表2-3同。
Note:Different lowercase means significantly difference at P=0.05;different capital letter means significantly difference at P=0.01. The same as Tab.2-3.

A.植株 Bar=15 cm;B.茎秆 Bar=20 cm;
C.节间 Bar=3 cm;D.果穗 Bar=3 cm;E.籽粒 Bar=1 cm。
A.Plants bar=15 cm;B.Stem bar=20 cm;C.Internode bar=3 cm;
D.Ear bar=3 cm;E.Kernel bar=1 cm.
图1 K718与K718d植株、茎秆、节间、果穗和籽粒对比
Fig.1 Comparisons of plants,stem,internode,
ears and kernels between the K718 and K718d
2.2 突变体K718d对GA3和IAA的敏感性
经2种外源激素处理后方差分析结果,除内源GA3在材料间差异不显著外,其余性状在材料间和浓度间差异均达极显著水平,材料与浓度互作间差异不显著。K718d与K718比较,低浓度GA3处理下第一叶鞘长度差异不显著,高浓度下差异极显著,而各浓度处理苗高均达极显著差异,但内源赤霉素含量(以鲜质量计)差异不显著(表2);不同IAA浓度处理,2个材料间第一叶鞘长度和苗高均达极显著差异(表3)。2种激素处理,均不能使突变体苗高恢复至野生型水平,说明该突变体对GA3和IAA不敏感,但K718d能够正常合成赤霉素,且转运GA3途径正常。
表2 K718d和K718第一叶鞘长度、苗高、内源赤霉素含量的多重比较 (GA3)
Tab.2 Multiple comparisons of the first sheath length,seeding height,
concentrations of endogenous GA3 between K718d and K718
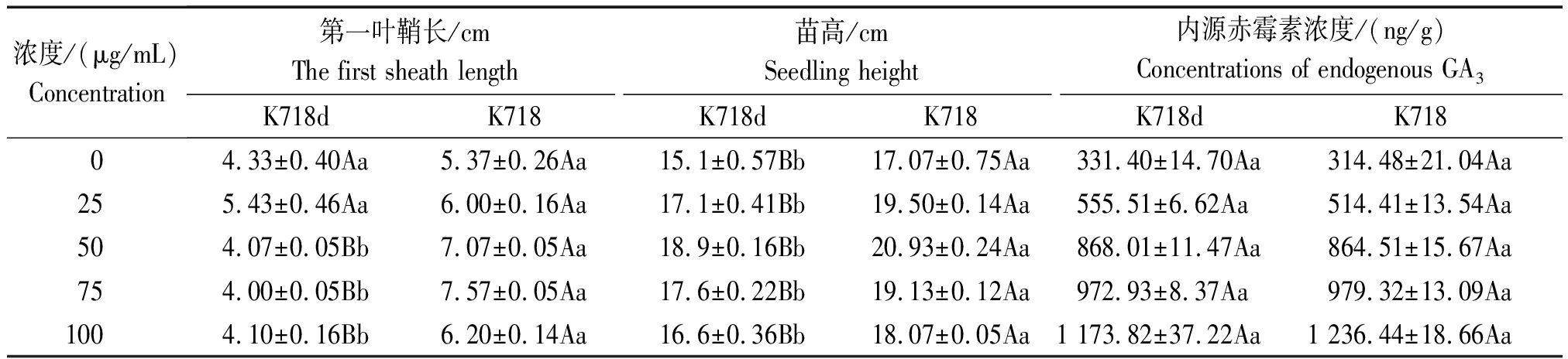
浓度/(μg/mL)Concentration第一叶鞘长/cmThe first sheath length苗高/cmSeedling height内源赤霉素浓度/(ng/g)Concentrations of endogenous GA3K718dK718K718dK718K718dK71804.33±0.40Aa5.37±0.26Aa15.1±0.57Bb17.07±0.75Aa331.40±14.70Aa314.48±21.04Aa255.43±0.46Aa6.00±0.16Aa17.1±0.41Bb19.50±0.14Aa555.51±6.62Aa514.41±13.54Aa504.07±0.05Bb7.07±0.05Aa18.9±0.16Bb20.93±0.24Aa868.01±11.47Aa864.51±15.67Aa754.00±0.05Bb7.57±0.05Aa17.6±0.22Bb19.13±0.12Aa972.93±8.37Aa979.32±13.09Aa1004.10±0.16Bb6.20±0.14Aa16.6±0.36Bb18.07±0.05Aa1 173.82±37.22Aa1 236.44±18.66Aa
表3 K718d和K718第一叶鞘长度和苗高的多重比较(IAA)
Tab.3 Multiple comparisons of the first sheath length and seeding height between k718d and K718(IAA)
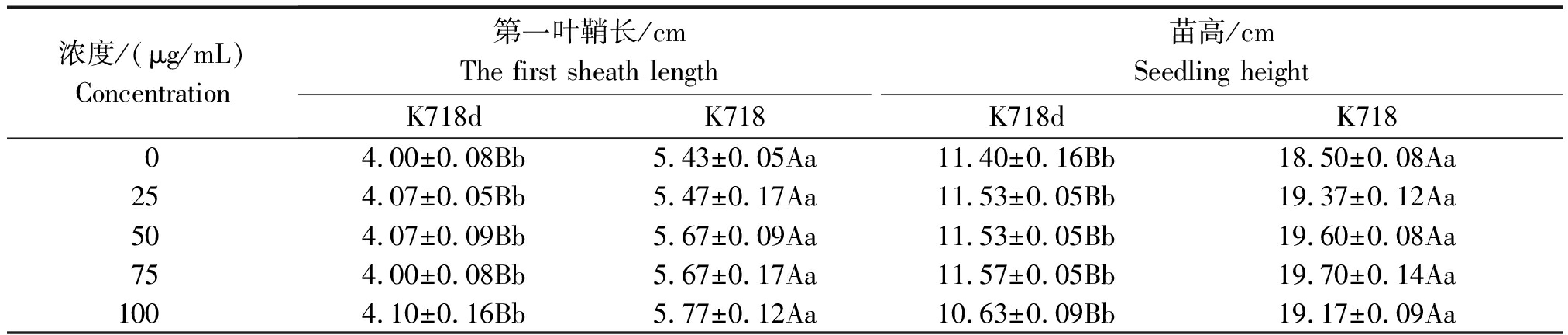
浓度/(μg/mL)Concentration第一叶鞘长/cmThe first sheath length苗高/cmSeedling heightK718dK718K718dK71804.00±0.08Bb5.43±0.05Aa11.40±0.16Bb18.50±0.08Aa254.07±0.05Bb5.47±0.17Aa11.53±0.05Bb19.37±0.12Aa504.07±0.09Bb5.67±0.09Aa11.53±0.05Bb19.60±0.08Aa754.00±0.08Bb5.67±0.17Aa11.57±0.05Bb19.70±0.14Aa1004.10±0.16Bb5.77±0.12Aa10.63±0.09Bb19.17±0.09Aa
2.3 突变体K718d株高遗传模式
2.3.1 正反交F1群体株高表现 将K718d配制的5个正反交群体平均株高列于表4。所有正反交F1在两试验点均表现为高秆,经t检验正反交F1群体平均株高差异不显著,初步说明株高无细胞质效应。
表4 正反交F1群体株高
Tab.4 Plant height of reciprocal F1 populations
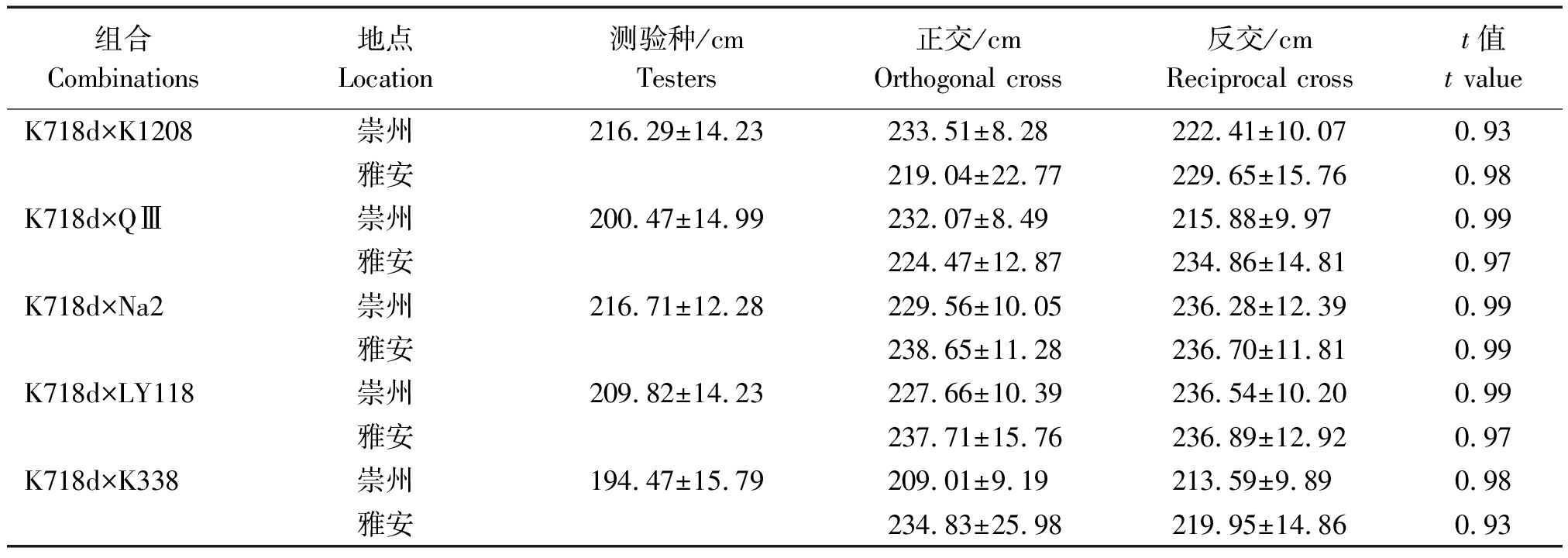
组合Combinations地点Location测验种/cmTesters正交/cmOrthogonal cross反交/cmReciprocal crosst值t valueK718d×K1208崇州216.29±14.23233.51±8.28222.41±10.070.93雅安219.04±22.77229.65±15.760.98K718d×QⅢ崇州200.47±14.99232.07±8.49215.88±9.970.99雅安224.47±12.87234.86±14.810.97K718d×Na2崇州216.71±12.28229.56±10.05236.28±12.390.99雅安238.65±11.28236.70±11.810.99K718d×LY118崇州209.82±14.23227.66±10.39236.54±10.200.99雅安237.71±15.76236.89±12.920.97K718d×K338崇州194.47±15.79209.01±9.19213.59±9.890.98雅安234.83±25.98219.95±14.860.93
2.3.2 回交群体株高分离比例 以K718d为回交亲本构建的BC1回交群体,在两生态点高秆与矮秆植株分离比例经卡方检验均符合1∶1(表5);而以5个测验种为回交亲本的BC2回交群体在两试验点均表现为高秆,表明K718d矮秆性状可能由1对隐性核基因控制。
表5 BC1分离群体株高χ2检验
Tab.5 The χ2 test of plant height of BC1 segregation populations
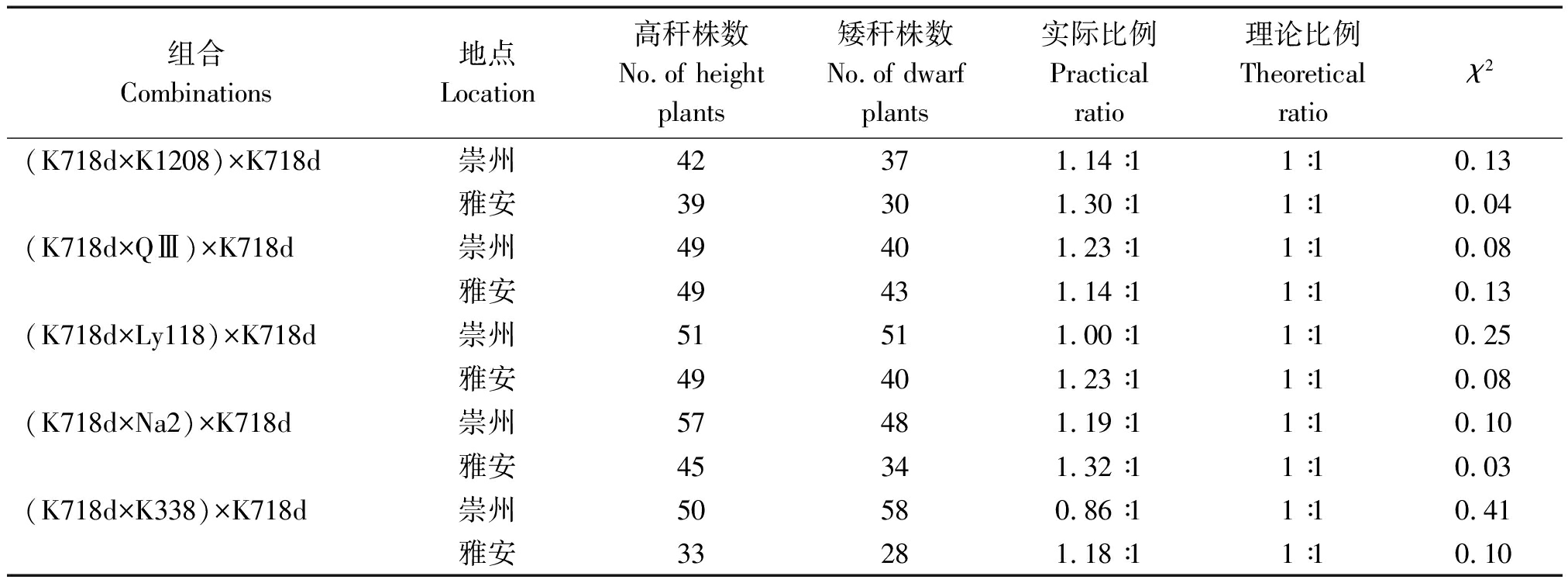
组合Combinations地点Location高秆株数No.of height plants矮秆株数No.of dwarf plants实际比例Practical ratio理论比例Theoretical ratioχ2(K718d×K1208)×K718d崇州42371.14∶11∶10.13雅安39301.30∶11∶10.04(K718d×QⅢ)×K718d崇州49401.23∶11∶10.08雅安49431.14∶11∶10.13(K718d×Ly118)×K718d崇州51511.00∶11∶10.25雅安49401.23∶11∶10.08(K718d×Na2)×K718d崇州57481.19∶11∶10.10雅安45341.32∶11∶10.03(K718d×K338)×K718d崇州50580.86∶11∶10.41雅安33281.18∶11∶10.10
2.3.3 F2群体株高分离比例 在2个生态点所有F2群体高秆与矮秆植株分离比例,经卡方测验都符合3∶1,进一步证明K718d的矮秆性状由1对隐性核基因控制(表6)。
表6 F2分离群体株高χ2检验
Tab.6 The χ2 test of plant height of F2 segregation populations
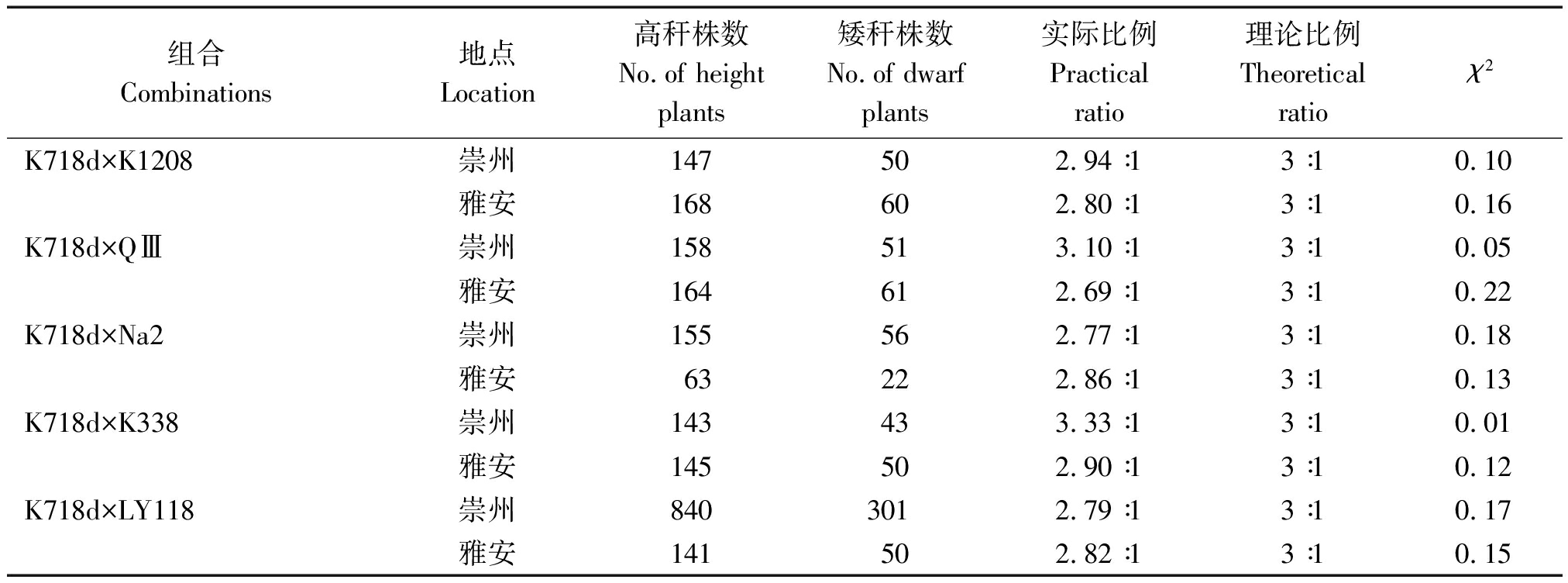
组合Combinations地点Location高秆株数No.of height plants矮秆株数No.of dwarf plants实际比例Practical ratio理论比例Theoretical ratioχ2K718d×K1208崇州147502.94∶13∶10.10雅安168602.80∶13∶10.16K718d×QⅢ崇州158513.10∶13∶10.05雅安164612.69∶13∶10.22K718d×Na2崇州155562.77∶13∶10.18雅安63222.86∶13∶10.13K718d×K338崇州143433.33∶13∶10.01雅安145502.90∶13∶10.12K718d×LY118崇州8403012.79∶13∶10.17雅安141502.82∶13∶10.15
综上所述,K718d与5个测验系组配的正反交F1在崇州和雅安两地均表现为高秆,其正反交组合株高差异不显著,无细胞质效应,受环境影响较小;K718d与F1构建的BC1群体高矮秆植株分离比符合1∶1,5个测验系与F1构建的BC2群体都表现为高秆,F2群体高矮秆植株分离比符合3∶1,表明K718d矮秆性状受一对细胞核隐性基因控制,暂将其命名为d718。
2.4 矮秆基因d718定位
定位群体为K718d×Ly118 F2,选取458对均匀覆盖于玉米10条染色体上的SSR引物,在双亲之间筛选出了170对多态性较好的引物后,继续在高、矮秆基因池间筛选,筛出1对多态性引物umc1446;以该引物为参考,继续合成1号染色体Bin1.06~1.09区段的45对引物进行多态性筛选,筛选出了6对多态性引物,至此共筛选出7对多态性引物(表7)。图2为7对多态性引物在F2群体高、矮秆基因池间的扩增结果。
表7 7对SSR引物序列信息
Tab.7 The sequence information of 7 pairs of SSR primers
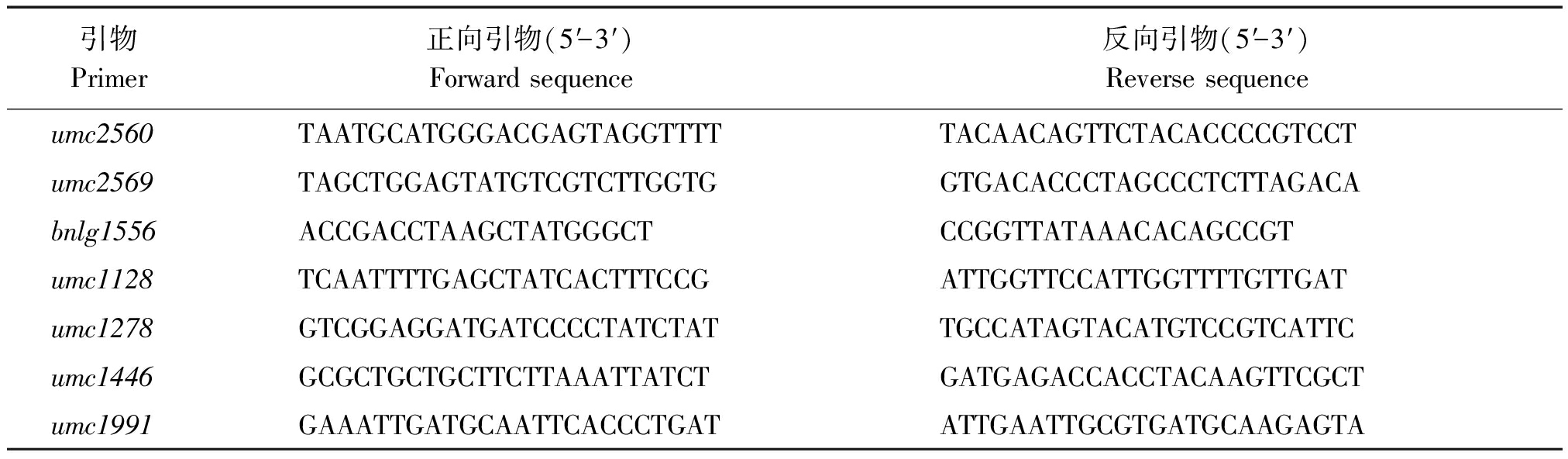
引物Primer正向引物(5′-3′)Forward sequence反向引物(5′-3′)Reverse sequence umc2560TAATGCATGGGACGAGTAGGTTTTTACAACAGTTCTACACCCCGTCCTumc2569TAGCTGGAGTATGTCGTCTTGGTGGTGACACCCTAGCCCTCTTAGACAbnlg1556ACCGACCTAAGCTATGGGCTCCGGTTATAAACACAGCCGTumc1128TCAATTTTGAGCTATCACTTTCCGATTGGTTCCATTGGTTTTGTTGATumc1278GTCGGAGGATGATCCCCTATCTATTGCCATAGTACATGTCCGTCATTCumc1446GCGCTGCTGCTTCTTAAATTATCTGATGAGACCACCTACAAGTTCGCTumc1991GAAATTGATGCAATTCACCCTGATATTGAATTGCGTGATGCAAGAGTA
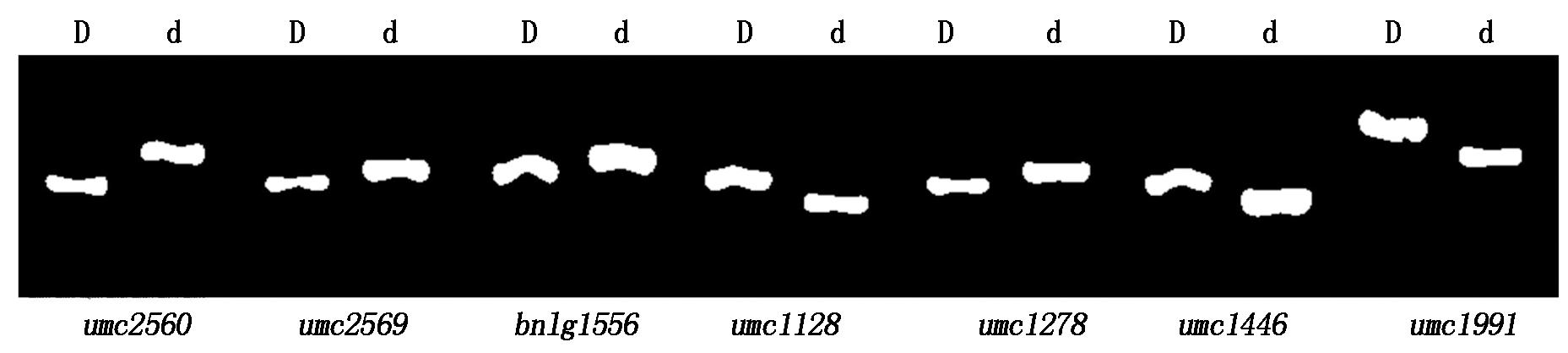
D.高秆基因池;d.矮秆基因池。
D.High stalk gene pools;d.Dwarf gene pools.
图2 7对多态性SSR引物在高矮秆基因池间的扩增结果
Fig.2 Amplified results of 7 polymorphism SSR primers between high and dwarf gene pools
用上述7对引物在K718d×Ly118的F2群体的316个矮秆单株分别进行PCR扩增,用3%琼脂糖凝胶进行扩增产物的电泳检测。用上述作图软件进行扩增结果连锁分析后,绘制出遗传连锁图谱。最终将该矮秆基因d718,初步定位于玉米1号染色体长臂上,位于SSR分子标记umc1128与umc1278之间,遗传距离分别为1.0,2.5 cM(图3)。
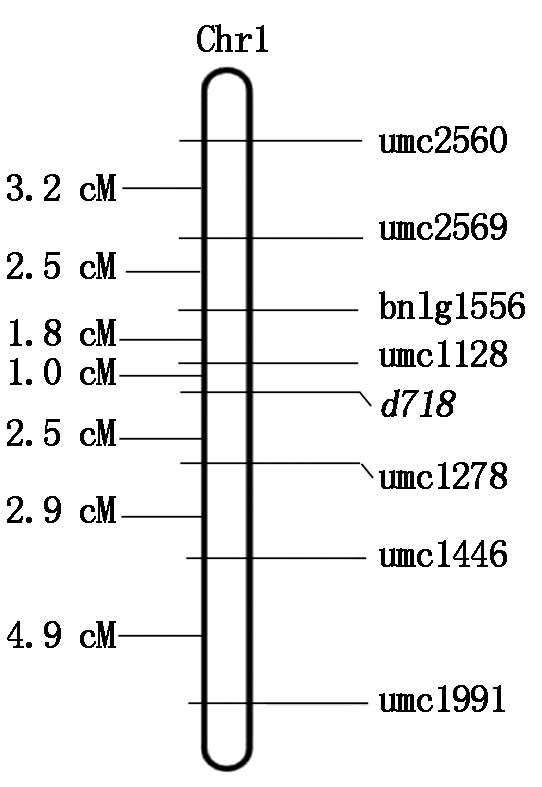
图3 矮秆基因d718遗传连锁图谱
Fig.3 Genetic linkage map of dwarf gene d718
2.5 d718基因的等位性鉴定
由于矮秆基因d718在染色体上与br1(1.07)、br2(1.06)等已知矮秆基因相近,有必要对该基因进行等位性鉴定。K718d与5个矮秆测验系组配的F1群体株高统计发现,除K718d×110K株高表现为矮秆外,其他组合均表现为高秆(表8)。在5个矮秆测验系中,已知110K为br1材料,301为cr1材料,其余3个均为br2突变体,试验结果只有K718d与110K杂交F1群体植株为矮秆,由此表明d718为br1的等位基因。
表8 F1群体株高
Tab.8 Plant height of F1 populations
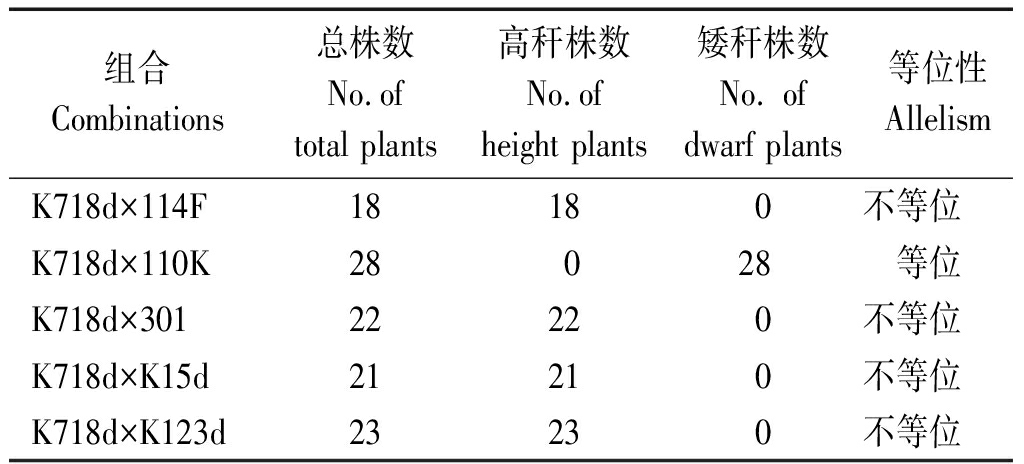
组合Combinations总株数No.oftotal plants高秆株数No.ofheight plants矮秆株数No. ofdwarf plants等位性AllelismK718d×114F18180不等位K718d×110K28028 等位K718d×30122220不等位K718d×K15d21210不等位K718d×K123d23230不等位
3 讨论与结论
3.1 矮秆突变体K718d表型特征
玉米茎秆节间数减少,节间长度变短可以造成株高的矮化,植物内源激素通过促进、抑制或改变生理活动,调控植物生长发育进程,参与植物株高建成[10-12]。前人研究表明,突变体A2节间数减少,节间长度缩短,dm676节间极显著缩短,从而导致植株矮化[13-14],br1类矮秆突变体节间显著缩短,平均单株产量只有野生型的2/3[15],且br1对GA3不敏感,而突变体523333[16]、d0掖(478)、d0(齐319)和d0(PH4CV)[17]对GA3敏感。
本研究发现,与野生型K718比较,K718d节间数减少近1/3,节间长度缩短近2/3,株高降低近1/2,平均单株产量降低36.44%,但穗粗增加8.31%,可用来改良玉米品种株高和穗型等性状,具有一定的应用价值。经不同浓度的GA3和IAA处理发现,该突变体为赤霉素与生长素钝感型,与矮秆突变体br1 和br2相似,但赤霉素合成及转运途径正常。推测造成突变体K718d矮化的原因,可能是植株生长过程中某一特定时期激素合成或运输有关,有待进一步研究。
3.2 矮秆突变体K718d株高遗传特性
现有研究发现,玉米株高分为单基因遗传和多基因遗传2种模式,其中单基因遗传又分为显性和隐性2种[18-19]。王立静等[20]和戚洪源等[21]利用矮秆突变体与普通玉米自交系构建F1、BC和F2群体的方法,分析明确了矮生性状的遗传模式。王益军等[22]发现了来自玉米自交系Mo17的一个显性矮秆突变基因D*-10,并采用 SSR分子标记技术,将该基因定位在玉米2号染色体上。王立静等[20]把121C(D8)和502C(D9)的花粉授予玉米矮秆显性突变体52333,取30株F1的花粉授予高秆自交系Lx9801,根据后代高矮秆植株分离比例,确定了Dt与D8和D9为非等位基因。
本研究用K718d与5个高秆自交系组配正反交F1、BC1、BC2和F2群体,分析矮秆性状的遗传模式,结果发现,K718d矮秆性状由1对隐性核基因控制;并利用BSA-SSR分子标记技术,将矮秆基因d718定位于1号染色体长臂上,位于分子标记umc1128与umc1278之间,遗传距离分别为1.0,2.5 cM;等位性鉴定发现,K718d与110K(br1)组配的F1群体株高表现为矮秆,表明d718与br1等位。后续将进一步对d718进行精细定位和克隆等研究,为育种应用提供技术支撑。
[1] Multani D S,Briggs S P,Chamberlin M A,Blakeslee J J,Murphy A S,Johal G S.Loss of an MDR transporter in compact stalks of maize br2 and Sorghum dw3 mutants[J].Science,2003,302(5642):81-84.doi:10.1126/science.1086072.
[2] Winkler R G,Helentjaris T. The maize Dwarf3 gene encodes a cytochrome P450-mediated early step in gib-berellin biosynthesis[J].The Plant Cell,1995,7(8):1307-1317.doi:10.1105/tpc.7.8.1307.
[3] Peng J R,Richards D E,Hartley N M,Murphy G P,Devos K M,Flintham J E,Beales J,Fish L J,Worland A J,Pelica F,Sudhakar D,Christou P,Snape J W,Gale M D,Harberd N P.Green revolution′genes encode mutant gibberellin response modulators[J].Nature,1999,400(6741):256-261.doi:10.1038/22307.
[4] Lawit S J,Wych H M,Xu D P,Kundu S M,Tomes D T.Maize DELLA proteins dwarf plant8 and dwarf plant9 as modulators of plant development[J].Plant and Cell Physiology,2010,51(11):1854-1868.doi:10.1093/pcp/pcq153.
[5] 王立静.玉米矮秆基因Dt和坏死基因nec-t的图位克隆与功能分析[D].泰安:山东农业大学,2012.doi:10.7666/d.d224451.
Wang L J.Map-based cloning and functional analysis of dwarf gene Dt and necrotic gene nec-t in maize[D].Taian:Shandong Agricultural University,2012.
[6] 何川,郑祖平,谢树果,李钟,刘代惠.隐性单基因br-2玉米矮生系的选育[J].中国农业科学,2009,42(8):2978-2981.doi:10.3864/j.issn.0578-1752.2009.08.042.
He C,Zheng Z P,Xie S G,Li Z,Liu D H.Breeding of the maize monogenic br-2 dwarf lines[J].Scientia Agricultura Sinica,2009,42(8):2978-2981.
[7] 徐敏,石海春,余学杰,谭义川,柯永川,赵长云,柯永培.一个玉米矮秆突变体K123d的遗传鉴定[J].植物遗传资源学报,2017,18(1):155-163.doi:10.13430/j.cnki.jpgr.2017.01.020.
Xu M,Shi H C,Yu X J,Tan Y C,Ke Y C,Zhao C Y,Ke Y P.Genetic identification of A dwarf mutant K123d in maize(Zea mays L.)[J].Journal of Plant Genetic Resources,2017,18(1):155-163.
[8] Michelmore R W,Paran I,Kesseli R V.Identification of markers linked to disease-resistance genes by bulked segregant analysis:A rapid method to detect markers in specific genomic regions by using segregating populations[J].Proceedings of the National Academy of Sciences of the United States of America,1991,88(21):9828-9832.doi:10.1073/pnas.88.21.9828.
[9] 刘仁虎,孟金陵.MapDraw、在 Excel 中绘制遗传连锁图的宏[J].遗传,2003,25(3):317- 321.doi:10.16288/j.yczz.2003.03.017.
Liu R H,Meng J L.MapDraw:a microsoft excel macro for drawing genetic linkage maps based on given genetic linkage data[J].Hereditas,2003,25(3):317-321.
[10] Wang L,Mu C,Du M W,Chen Y,Tian X L,Zhang M C,Li Z H.The effect of mepiquat chloride on elongation of cotton(Gossypium hirsutum L.)internode is associated with low concentration of gibberellic acid[J].Plant Science,2014,225:15-23.doi:10.1016/j.plantsci.2014.05.005.
[11] Srinivasan C,Liu Z R,Scorza R.Ectopic expression of class 1 KNOX genes induce adventitious shoot regeneration and alter growth and development of tobacco(Nicotiana tabacum L)and European plum(Prunus domestica L)[J].Plant Cell Reports,2011,30(4):655-664.doi:10.1007/s00299-010-0993-7.
[12] Agehara S,Leskovar D I.Age-dependent effectiveness of exogenous abscisic acid in height control of bell pepper and ![]() transplants[J].Scientia Horticulturae, 2014,175:193-200.doi:10.1016/j.scienta.2014.05.025.
transplants[J].Scientia Horticulturae, 2014,175:193-200.doi:10.1016/j.scienta.2014.05.025.
[13] 董春林,翟广谦,张正,杨睿,张明义,张彦琴,杨丽莉,常建忠.玉米矮秆突变体A2的表型鉴定及转录组分析[J].玉米科学,2019,27(4):52-57.doi:10.13597/j.cnki.maize.science.20190408.
Dong C L,Zhai G Q,Zhang Z,Yang R,Zhang M Y,Zhang Y Q,Yang L L,Chang J Z.Phenotypic characterization and transcriptome analysis of maize dwraf mutant A2[J].Journal of Maize Sciences,2019,27(4):52-57.
[14] 邱正高,杨华,袁亮,张亚勤,张采波,汤玲,荣廷昭,曹墨菊.一份新选玉米矮秆突变体的鉴定与遗传分析[J].华北农学报,2015,30(6):112-118.doi:10.7668/hbnxb.2015.06.017.
Qiu Z G,Yang H,Yuan L,Zhang Y Q,Zhang C B,Tang L,Rong T Z,Cao M J.Identification and genetic analysis of a new dwarf mutant in maize[J].Acta Agriculturae Boreali-Sinica,2015,30(6):112-118.
[15] Oh M H,Sun J D,Oh D H,Zielinski R E,Clouse S D,Huber S C.Enhancing Arabidopsis leaf growth by engineering the BRASSINOSTEROID INSENSITIVE1 receptor kinase[J].Plant Physiology,2011,157(1):120-131.doi:10.1104/pp.111.182741.
[16] 张素梅.玉米株高突变体的遗传分析和初步基因定位[D].泰安:山东农业大学,2008.doi:10.7666/d.y1374584.
Zhang S M.Genetic analysis and preliminary gene mapping of a plant height mutant in maize[D].Taian:Shandong Agricultural University,2008.
[17] 王武全,曹本高,员海燕.玉米矮秆突变体的激素敏感性分析[J].西北农林科技大学学报(自然科学版),2017,45(8):51-55.doi:10.13207/j.cnki.jnwafu.2017.08.008.
Wang W Q,Cao B G,Yun H Y.Hormone sensitivity of a dwarf mutant of maize[J].Journal of Northwest A&F University (Natural Science Edition),2017,45(8):51-55.
[18] 李钟,郑祖平,张国清,何川.矮生玉米自交系的选育和利用[J].玉米科学,2006,14(1):76-78.doi:10.3969/j.issn.1005-0906.2006.01.023.
Li Z,Zheng Z P,Zhang G Q,He C.Breeding and application on the inbred line of dwarf maize[J].Journal of Maize Sciences,2006,14(1):76-78.
[19] 李忠南,王克伟,王越人,邬生辉,李光发.玉米品种先玉335的血缘系谱及主要农艺性状遗传分析[J].玉米科学,2018,26(3):32-39.doi:10.13597/j.cnki.maize.science.20180307.
Li Z N,Wang K W,Wang Y R,Wu S H,Li G F.Genetic analysis on pedigree and agronomic characters of maize variety Xianyu 335[J].Journal of Maize Sciences,2018,26(3):32-39.
[20] 王立静,哈丽旦,张素梅,徐春花,刘保申.新的玉米矮秆突变基因的鉴定与遗传分析[J].华北农学报,2008,23(5):23-25.doi:10.7668/hbnxb.2008.05.005.
Wang L J,Ha L D,Zhang S M,Xu C H,Liu B S.Identification and genetic analysis of a new dwarf mutant gene in maize[J].Acta Agriculturae Boreali-Sinica,2008,23(5):23-25.
[21] 戚洪源,李卫华,付志远,丁冬,胡彦民,汤继华.玉米隐性矮秆突变体的遗传分析与初步定位[J].河南农业大学学报,2013,47(3):245-249.doi:10.3969/j.issn.1000-2340.2013.03.003.
Qi H Y,Li W H,Fu Z Y,Ding D,Hu Y M,Tang J H.Genetic analysis and linkage mapping of a recessive dwarf mutant in maize[J].Journal of Henan Agricultural University,2013,47(3):245-249.
[22] 王益军,苗楠,施亚婷,邓德祥,卞云龙.一份玉米显性矮秆突变体的遗传分析[J].华北农学报,2010,25(5):90-93.doi:10.7668/hbnxb.2010.05.019.
Wang Y J,Miao N,Shi Y T,Deng D X,Bian Y L.Genetic analysis of a dominant dwarf mutant in maize[J].Acta Agriculturae Boreali-Sinica,2010,25(5):90-93.