油用向日葵(Helianthus annuus L.)是世界上植物油的重要来源,油用向日葵贡献了近3.3×107 t的油籽产量,占世界总产量的8.5%[1]。我国是世界上重要的油用向日葵生产国和消费国之一,其中以内蒙古河套地区的种植面积和总产量居于首位。河套地区多年平均降水量为130~150 mm,蒸发量2 000~3 000 mm,属于全国地表水资源缺乏的地区;加之近些年黄河灌溉水量配额下调的原因,农业灌溉用水常显不足,水资源短缺势必将成为今后该地区油用向日葵生产的限制因子。非生物胁迫是世界上作物生产的主要限制因素[2-3],因干旱胁迫每年约有超过30%的生产用地面临减产,干旱造成的作物减产已超过其他逆境造成减产的总和[4]。有研究表明,干旱胁迫会干扰向日葵的生理过程和生化活性进而影响向日葵的生长速度[5],并导致叶面积指数、叶片相对含水量、生物产量、收获指数以及油籽产量降低[6]。
随着现代分子生物学技术在植物逆境研究领域的应用[7],特别是高通量测序技术的应用,可以通过表型与转录组联合分析用于阐明与水分胁迫反应有关的基因调控网络[8]。已有研究表明,WRKY[9]、ERF[10]和NAC[11]等转录因子在非生物胁迫条件下对植株抗逆性有一定作用。干旱胁迫下会引发植物的应激反应,如激素水平上的变化、气孔关闭及功能基因的表达等[12],这其中以脱落酸(ABA)被广泛关注,ABA在植物应对水分胁迫的能力中起主要作用[13]。王芳等[14]研究表明,干旱胁迫下外源ABA对玉米幼苗氧化损伤有保护作用。在缺水条件下,ABA作为一种重要的根源信号物质,通过木质部的运输和蒸腾到达叶片保卫细胞并发出信号,表明植物根部正处于压力状态,进而使蒸腾速率降低,叶片保卫细胞的膨压下降,最终引起气孔关闭[15]。脱落酸信号转导首先需要识别脱落酸才能进行后续的过程,PYR/PYR/RCAR蛋白被证明是ABA受体之一,处于ABA信号转导通路的最上游,可以识别并结合ABA,且PP2C蛋白磷酸酶家族对ABA应答起调控作用[16]。本研究以油用向日葵稳定自交系为材料,研究了持续干旱和复水处理下的ABA表达水平,探讨了脱落酸的变化与干旱胁迫的关系,以期为激素代谢差异基因的挖掘及激素与其他代谢途径的互作关系提供理论依据。
1 材料和方法
1.1 供试材料
试验材料为稳定自交系17062(9D002),由内蒙古农业大学农学院油用向日葵研究室提供。
1.2 试验设计
试验于内蒙古农业大学农学院(40°48′N,111°42′E)日光温室中进行,采用盆栽方式进行干旱胁迫,以田间土∶蛭石∶营养土=1∶1∶1为栽培土壤,每盆5 kg,共40盆,选取饱满、无病害的油用向日葵种子,每盆定植4株。每组分别设置正常供水(CK)和控水(T)处理各20盆。在生长至第5对真叶时开始进行控水胁迫处理,胁迫天数分为控水0,4,8(TAR),12,16 d(TAX),胁迫16 d后进行复水(FS),胁迫期间对照组正常浇水,正常浇水8 d为CKAR、16 d为CKAX。测定控水时期的ABA含量并对控水8,16 d时进行转录组测序。
1.3 脱落酸含量的测定
在干旱胁迫各个时期对叶片进行混合取样,置于-20 ℃冰箱保存,采用酶联免疫吸附法测定脱落酸含量[17]。
1.4 总RNA的提取、文库的构建及测序
提取样品总RNA并使用DNase消化DNA后,用带有Oligo(dT)的磁珠富集真核生物mRNA(若为原核生物,则通过试剂盒去除rRNA来富集mRNA);加入打断试剂将mRNA打断成短片段,以打断后的mRNA为模板,用六碱基随机引物合成一链cDNA,然后配制二链成反应体系合成二链cDNA,并使用试剂盒纯化双链cDNA;纯化的双链cDNA再进行末端修复、加A尾并连接测序接头,然后进行片段大小选择,最后进行PCR扩增;构建好的文库用Agilent 2100 Bioanalyzer质检合格后,用Illumina HiSeqTM 2500或Illumina HiSeq X Ten等测序仪进行测序,产生125 bp或150 bp的双端数据。质检合格后,使用Illumina测序仪进行测序。建库测序由上海欧易生物医学科技有限公司完成。
2 结果与分析
2.1 油用向日葵叶片脱落酸含量对干旱胁迫的响应
干旱胁迫下,油用向日葵叶片脱落酸含量变化见图1。干旱胁迫8 d与16 d处理间存在显著差异,且在干旱胁迫8 d的脱落酸含量最高,为23.55 ng/mL,在干旱胁迫16 d脱落酸含量最低,为15.74 ng/mL,干旱胁迫4 d比0 d增加2.38%,8 d比4 d增加19.86%,12 d比8 d降低25.33%,16 d比12 d降低10.55%。干旱胁迫4,8 d较0 d升高,随着干旱胁迫时间的延长及植物的生长发育,脱落酸含量呈先增加后降低的趋势,说明植物遭受适当干旱胁迫时,植物会通过增加脱落酸含量来抵御干旱胁迫,而受到重度干旱胁迫时,脱落酸含量下降,干旱胁迫复水后脱落酸含量又有所升高,说明干旱胁迫给植物造成损伤后恢复到适宜的环境中,脱落酸含量会有一定程度的恢复。
2.2 差异基因筛选及富集分析
对差异表达基因(DEGs)以P<0.05且∣log2FC∣>1为条件进行筛选,筛选结果见表1。由表1、图2可知,在干旱胁迫8,16 d,通过与CK比较,处理组较CK显著上调表达的差异基因数分别为940,1 743个,显著下调表达的基因数分别为1 752,3 037个;在干旱胁迫16 d的上调及下调基因数较干旱胁迫8 d分别高85.43%,73.34%。由KEGG、GO功能注释可知(表2、图3),在干旱胁迫16 d较8 d的基因种类更丰富,其中参与生物过程的主要有生物调节、组织或生物合成、代谢过程等,参与细胞组分的主要有膜的组成部分、质膜等,参与分子功能的主要有结合、催化活性、转导活性等,KEGG注释到的主要代谢通路有植物MAPK信号通路途径、植物激素信号转导、核糖体等。
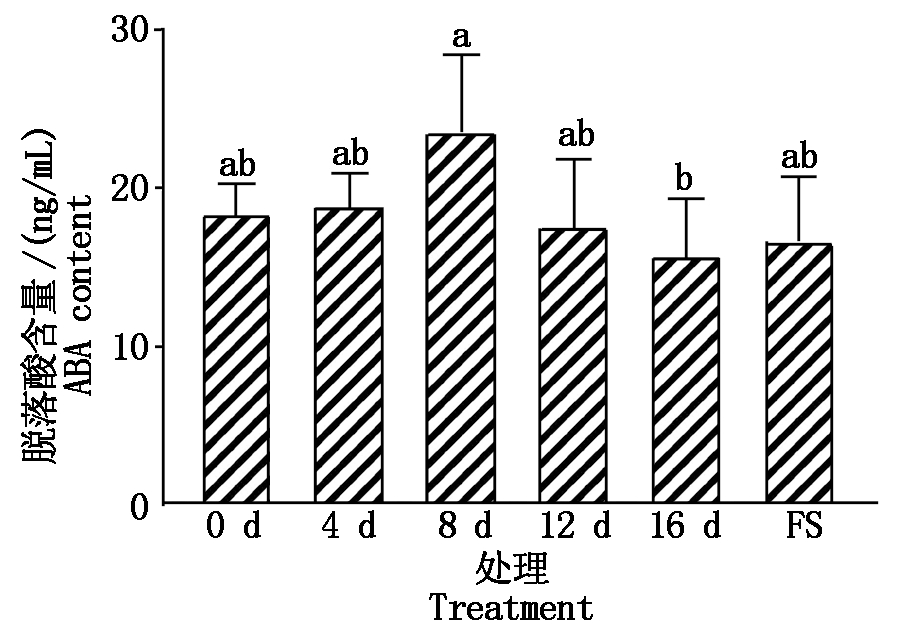
FS.复水处理;不同小写字母表示在0.05水平具有显著性差异。图5同。
FS.Rehydration treatment;Different lowercase letters
indicate significant differences at the 0.05 level.The same as Fig.5.
图1 干旱胁迫下叶片脱落酸含量差异
Fig.1 Difference of abscisic acid content in
leaves under drought stress
表1 差异表达基因筛选及各通路富集分类
Tab.1 Screening of differentially expressed genes and enrichment classification of waypath
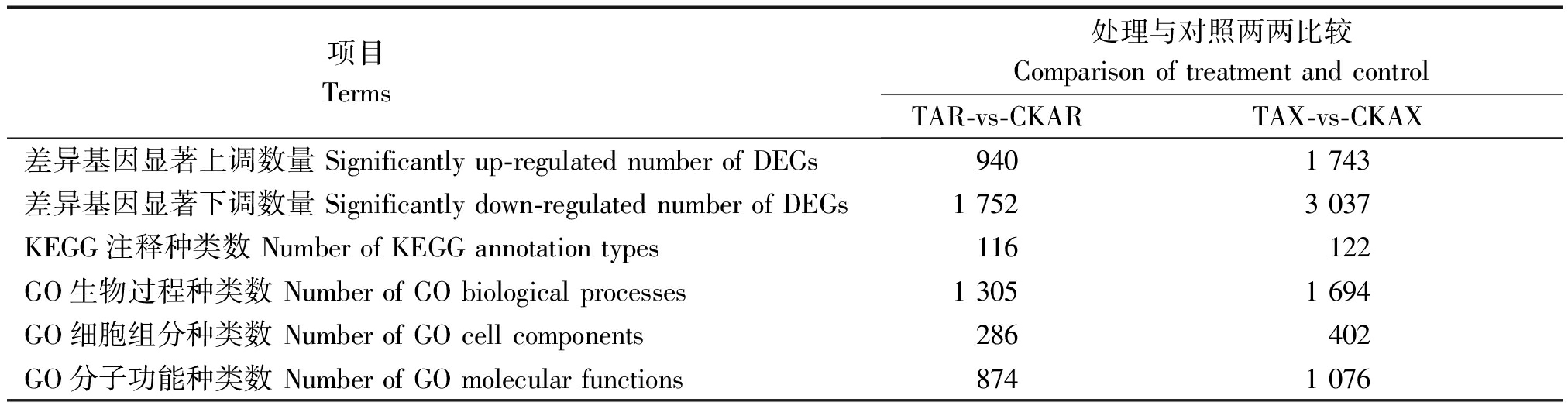
项目 Terms 处理与对照两两比较Comparison of treatment and controlTAR-vs-CKARTAX-vs-CKAX差异基因显著上调数量 Significantly up-regulated number of DEGs9401 743差异基因显著下调数量 Significantly down-regulated number of DEGs1 7523 037KEGG注释种类数 Number of KEGG annotation types116122GO生物过程种类数 Number of GO biological processes1 3051 694GO细胞组分种类数 Number of GO cell components286402GO分子功能种类数 Number of GO molecular functions8741 076
表2 主要KEGG代谢通路注释
Tab.2 Notes on the main KEGG metabolic pathways
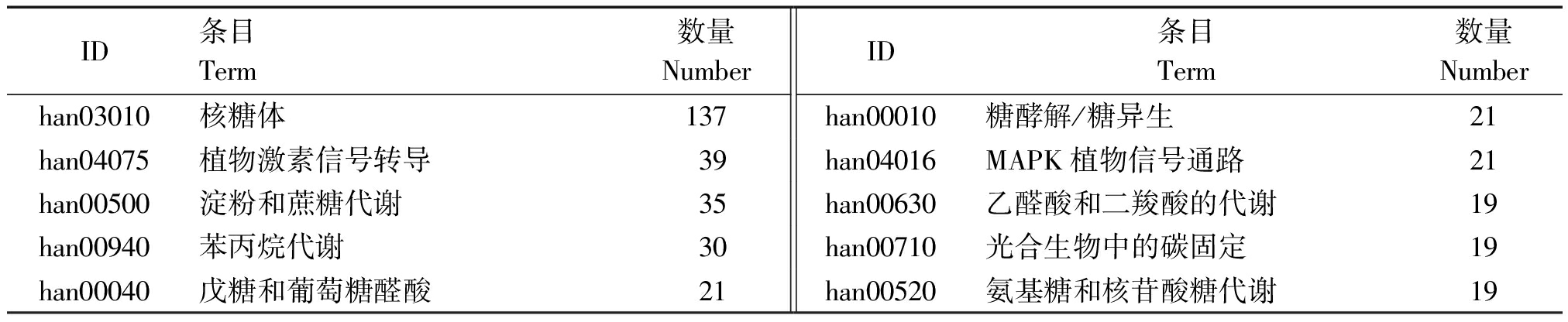
ID条目Term数量NumberID条目Term数量Numberhan03010核糖体137han00010糖酵解/糖异生21han04075植物激素信号转导39han04016MAPK植物信号通路21han00500淀粉和蔗糖代谢35han00630乙醛酸和二羧酸的代谢19han00940苯丙烷代谢30han00710光合生物中的碳固定19han00040戊糖和葡萄糖醛酸21han00520氨基糖和核苷酸糖代谢19
图2 干旱胁迫8 d(A)和16 d(B)差异基因上/下调
Fig.2 Up/down regulation of differential genes on the 8 d(A)and 16 d (B)of drought stress
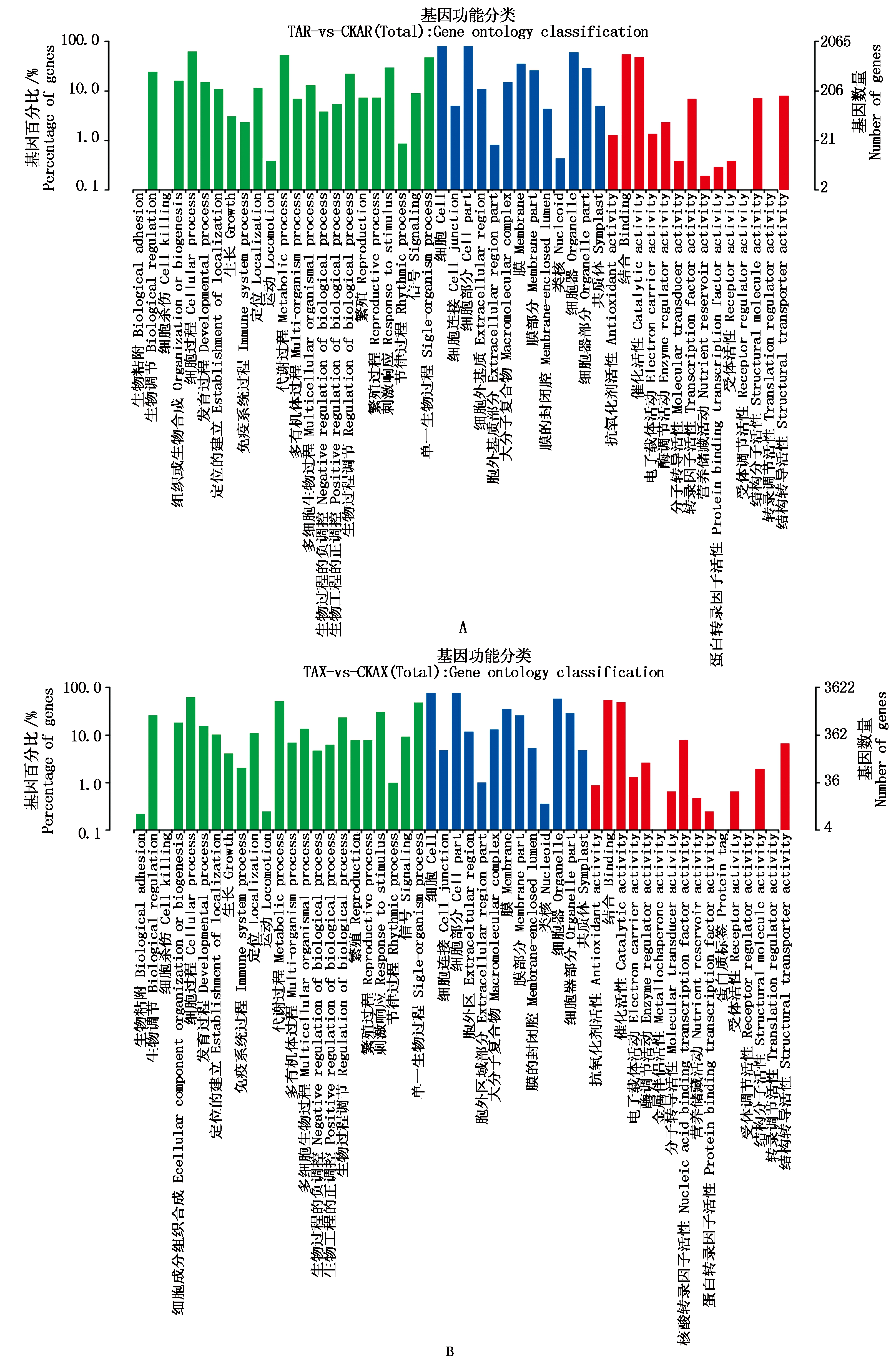
绿色.生物过程;蓝色.细胞组分;红色.分子功能。
Green.Biological processes;Blue.Cellular components;Red.Molecular functions.
图3 干旱胁迫8 d(A)和16 d(B)GO代谢通路富集
Fig.3 Enrichment of GO metabolic pathways on the 8 d(A)and 16 d(B)of drought stress
2.3 脱落酸代谢途径分析
通过对干旱胁迫8 d进行KEGG富集分析可知,与植物激素信号转导相关的基因共有39 个,分别为LOC110942898、LOC110928553、LOC110930956、LOC110934537、LOC110938235、LOC110940069、LOC110865531、LOC110868014、LOC110873889、LOC110874243、LOC110879426、LOC110880149、LOC110883801、LOC110886507、LOC110889226、LOC110889238、LOC110891642、LOC110890361、LOC110890646、LOC110893934、LOC110894171、LOC110894173、LOC110894172、LOC110894634、LOC110898754、LOC110899197、LOC110899792、LOC110908801、LOC110906168、LOC110906280、LOC110911693、LOC110911722、LOC110912152、LOC110912611、LOC110912722、LOC110916036、LOC110915273、LOC110916907、LOC110920591,其代谢途径如图4所示,其中表现上调的转录因子有SAUR、AHP、B-ARR、GID1、GID2、PYR/PYL、PP2C、SnRK2、ABF、ETR、ERF 1/2,ABA的核心通路为ABA→PYR/PYL→PP2C→SnRK2→ABF。在39个与植物激素信号转导的基因中有7个基因与脱落酸相关,分别为LOC110879426、LOC110883801、LOC110886507、LOC110898754、LOC110899197、LOC110899792、LOC110912152(表3),其中基因LOC110879426、LOC110899197干旱胁迫较对照差异显著,LOC110886507、LOC110898754干旱胁迫较对照差异极显著。

图4 植物激素信号转导代谢途径图
Fig.4 Diagram of the metabolic pathway of plant hormone signal transduction
表3 脱落酸差异基因表达分析
Tab.3 Analysis of differential gene expression of abscisic acid
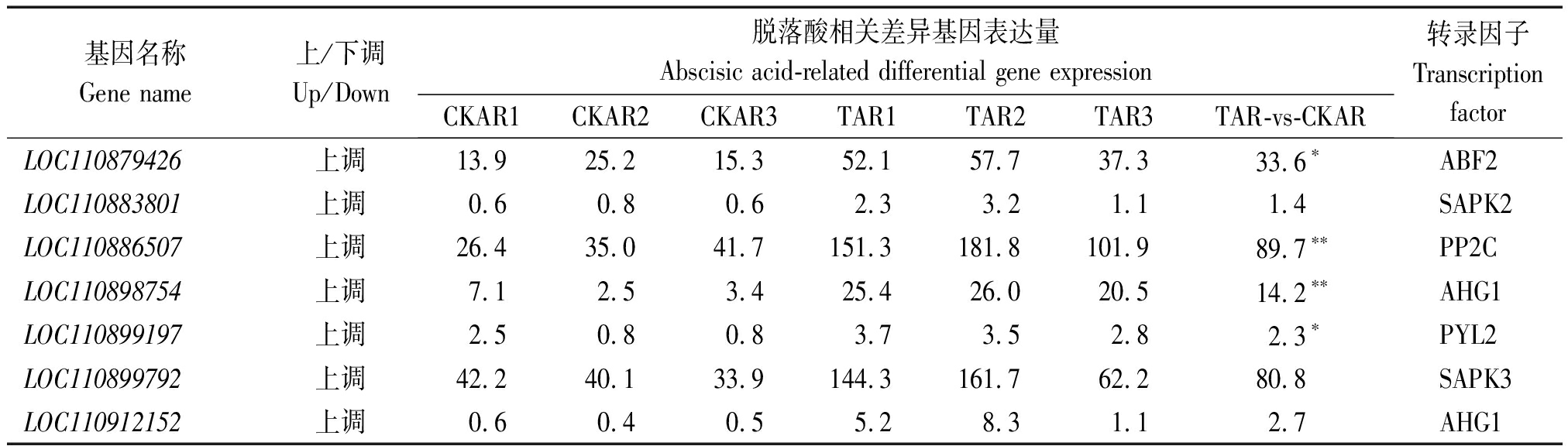
基因名称Gene name上/下调Up/Down脱落酸相关差异基因表达量Abscisic acid-related differential gene expressionCKAR1CKAR2CKAR3TAR1TAR2TAR3TAR-vs-CKAR转录因子TranscriptionfactorLOC110879426上调13.925.215.352.157.737.333.6∗ABF2LOC110883801上调0.60.80.62.33.21.11.4SAPK2LOC110886507上调26.435.041.7151.3181.8101.989.7∗∗PP2CLOC110898754上调7.12.53.425.426.020.514.2∗∗AHG1LOC110899197上调2.50.80.83.73.52.82.3∗PYL2LOC110899792上调42.240.133.9144.3161.762.280.8SAPK3LOC110912152上调0.60.40.55.28.31.12.7AHG1
注:*. 在0.05水平存在显著差异;**. 在0.01水平存在极显著差异。
Note:*. Significant difference at the 0.05 level; **. Extremely significant difference at the 0.01 level.
2.4 干旱胁迫热激蛋白变化
由图5可知,干旱胁迫过程中出现多种热激蛋白,其中HSP26在干旱胁迫8,16 d表达量较CK升高,且干旱胁迫16 d高于8 d,干旱胁迫第8,16天较各自对照呈显著性差异。HSP70在干旱胁迫8,16 d表达量低于CK,热激蛋白与脱落酸调控相关,因此,热激蛋白的变化可证明干旱胁迫下游用向日葵以脱落酸升高来适应和抵御逆境。

图5 干旱胁迫下热激蛋白的相对表达量
Fig.5 Relative expression of heat shock protein under drought stress
3 讨论与结论
可溶性糖、可溶性蛋白是重要的渗透调节物质,甜菜碱、脱落酸是调控逆境的激素类物质,在植物逆境中起着重要的作用[18]。在干旱胁迫过程中,植物的抗性表现受多种分子和细胞通路的调控,这其中就包括了糖代谢、激素代谢、脂代谢等代谢途径。对不同抗旱性小麦根系的转录组差异表达基因富集分析发现,抗性基因主要富集在植物的碳代谢、类黄酮合成及植物激素信号传导等途径[19]。研究表明,逆境诱导的内源激素脱落酸(ABA)可通过参与多重信号转导网络来调节植物的生理响应[20]。ABA调控胁迫主要有2条路径,一条依赖ABA,另一条则不依赖ABA;对于不依赖ABA的转导途径,植物将识别到的胁迫信号进行以Ca2+和干旱应答元件为中心的信号转导,从而诱导植物发生相应的生理生化变化以缓解逆境伤害[21],研究中发现基因表达的变化会引起表型的变异[22]。本研究中干旱胁迫第4,8天脱落酸含量间差异不显著,究其原因是植物在受到逆境胁迫时优先表现于分子水平的差异,而生理水平上的表达较缓慢,但各时期脱落酸含量较正常供水明显升高,复水后恢复至正常水平。经过对脱落酸相关基因表达量进行分析,发现受到干旱胁迫时脱落酸相关基因的表达量显著升高,故对干旱胁迫8 d的植物激素信号转导途径的差异基因进行分析,与脱落酸代谢相关的基因共7个,均为上调表达,且受到干旱胁迫的样本差异基因表达量均高于正常供水的样本。
植物受到胁迫刺激会诱导转录因子产生,转录因子会将信号传递,能够通过调控靶基因转录效率,从多个层面降低逆境胁迫对植物的伤害,对植物在逆境下的生长发育起到关键的调节作用[23-24],尤其是WRKY、MYB、NAC、bZIP等家族参与多种代谢途径,是信号通路上的关键调控因子[25-26]。本研究中干旱胁迫8 d处理与对照组对比,生理水平上处理组脱落酸含量高于正常供水,分子水平上ABF2、SAPK2、PP2C、AHG1、PYL2、SAPK3等转录因子编码的基因均呈现上调表达且表达量较高,在植物激素信号转导代谢途径中占主要比例,受到胁迫的处理基因表达量高于对照。王彬等[27]在干旱敏感材料中发现DEGs在植物激素信号转导通路有显著富集。本研究经两两对比发现,几类转录因子对干旱胁迫下植物激素信号转导代谢途径的调控作用极显著,其中,蛋白磷酸酶(PP)和bZIP的A亚族(ABF)参与脱落酸代谢[28],干旱胁迫下,植株体内脱落酸积累,ABA是植物抗旱的关键性调节因子,当受体PYR/PYL感知到ABA存在时,便相互结合,进一步与PP2C结合,形成三元复合体,抑制PP2C的酶活性,同时使PP2Cs-SnRK2复合体解离,SnRK2发生自磷酸化,随后通过磷酸化激活转录因子或离子通道等下游底物,诱导ABA响应基因表达或气孔关闭[29],与前人研究结果一致,ABF转录因子在番茄、烟草、葡萄及棉花中能增强植物应对非生物胁迫的能力[30-31]。ABA受体蛋白通过与逆境诱发的ABA结合,介导逆境信号传递,是特定渗透胁迫诱导的ABA信号传递通路重要组分[32]。部分ABA信号通路组分编码基因,通过在转录水平上对渗透胁迫逆境产生应答,对特定逆境信号进行传递[33]。如拟南芥PYR家族成员AtPYL的转录本丰度在干旱和盐分逆境下显著增大,通过基因转录效率改变,改善与ABA结合能力,对参与植株逆境响应的ABA信号通路产生调控[34]。
本研究以稳定自交系17062为材料对干旱胁迫8,16 d进行了RNA-seq测序及脱落酸含量测定,筛选出GID1、GID2、AHP、PYR/PYL、PP2C、SnRK2、ABF、ETR等与脱落酸相关的高表达差异基因,可能参与植株干旱调控,其中ABF、PYL等脱落酸受体,可以更好地结合脱落酸,而脱落酸含量在适当干旱胁迫下会升高,这说明脱落酸对植株抵御干旱逆境产生重要影响。在差异基因中筛选出热激蛋白(HSP26、HSP70)表达量较高,其中HSP26在GO功能注释到与脱落酸的调控相关,已有试验证明,ABA可调节热激蛋白(HSP)基因表达,促进其合成,从而提高植物抗逆能力[35],脱落酸差异基因表达变化与生理指标变化具有一致性,说明转录组数据具有可靠性。干旱胁迫下转录因子家族成员通过上调或下调表达来促进或抑制一些基因的表达水平以调节作物生长发育,从而减少干旱胁迫对植物正常生命活动的伤害。本研究对干旱胁迫下油用向日葵差异表达与脱落酸相关转录因子进行初步分析,下一步将对筛选出的差异基因进行验证,结果将为转录因子的共表达、干旱胁迫的调控网络及油用向日葵分子育种提供基础理论指导。
[1] Umar M,Uddin Z,Siddiqui Z S.Responses of photosynthetic apparatus in sunflower cultivars to combined drought and salt stress[J].Photosynthetica,2019,57(2):627-639.doi:10.32615/ps.2019.043.
[2] Wang W X,Vinocur B,Altman A.Plant responses to drought,salinity and extreme temperatures:Towards genetic engineering for stress tolerance[J].Planta,2003,218(1):1-14.doi:10.1007/s00425-003-1105-5.
[3] 齐容镰,莎仁图雅,李钢铁,李佳陶,何亮,杨文斌.干旱胁迫对小胡杨2号幼苗光合及生理特征的影响[J].干旱区研究,2020,37(6):1552-1561.doi:10.13866/j.azr.2020.06.21.
Qi R L,Sharen T Y,Li G T,Li J T,He L,Yang W B.Effects of drought stress on photosynthesis and physiological characteristics of Populus simonii×P.euphratica Xiaohuyang 2[J].Arid Zone Research,2020,37(6):1552-1561.
[4] 曲涛,南志标.作物和牧草对干旱胁迫的响应及机理研究进展[J].草业学报,2008,17(2):126-135.doi:10.3321/j.issn:1004-5759.2008.02.018.
Qu T,Nan Z B.Research progress on responses and mechanisms of crop and grass under drought stress[J].Acta Prataculturae Sinica,2008,17(2):126-135.
[5] Umar M,Siddiqui Z S.Physiological performance of sunflower genotypes under combined salt and drought stress environment[J].Acta Bot Croat,2018,77(1):36-44.doi:10.2478/botcro-2018-0002.
[6] Ghaffari M,Torchi M,Valizadeh M.Grain yield stabilizing physiological characteristics of sunflower under limited irrigation condition[J].Journal of Sustainable Agricultural and Production Science,2014,24:97-108.doi:10.1016/j.envexpbot.2014.08.005.
[7] 张小芳,乔亚科,王冰冰,徐燕,张锴,李桂兰.干旱胁迫下野生大豆ABC转运蛋白转录组测序分析[J].核农学报,2019,33(8):1474-1482.doi:10.11869/j.issn.100-8551.2019.08.1474.
Zhang X F,Qiao Y K,Wang B B,Xu Y,Zhang K,Li G L.Sequence analysis of ABC transporter transcriptome in wild soybean under the drought stress[J].Journal of Nuclear Agricultural Sciences,2019,33(8):1474-1482.
[8] Rengel D,Arribat S,Maury P,Martin-Magniette M L,Hourlier T,Laporte M,Varès D,Carrère S,Grieu P,Balzergue S,Gouzy J,Vincourt P,Langlade N B.A gene-phenotype network based on genetic variability for drought responses reveals key physiological processes in controlled and natural environments[J].PLoS One,2012,7(10): e45249.doi:10.1371/journal.pone.0045249.
[9] 黄胜雄,刘永胜.土豆WRKY转录因子家族的生物信息学分析[J].应用与环境生物学报,2013,19(2):205-214.doi:10.3724/SP.J.1145.2013.00205.
Huang S X,Liu Y S.Genome-wide analysis of WRKY transcription factors in Solanum tuberosum[J].Chinese Journal of Applied and Environmental Biology,2013,19(2):205-214.
[10] Charfeddine M,Saïdi M N,Charfeddine S,Hammami A,Gargouri Bouzid R.Genome-wide analysis and expression profiling of the ERF transcription factor family in potato(Solanum tuberosum L.)[J].Molecular Biotechnology,2015,57(4):348-358.doi:10.1007/s12033-014-9828-z.
[11] Singh A K,Sharma V,Pal A K,Acharya V,Ahuja P S.Genome-wide organization and expression profiling of the NAC transcription factor family in potato(Solanum tuberosum L.)[J].DNA Research,2013,20(4):403-423.doi:10.1093/dnares/dst019.
[12] Lim C W,Kim J H,Baek W,Kim B S,Lee S C.Functional roles of the protein phosphatase 2C,AtAIP1,in abscisic acid signaling and sugar tolerance in Arabidopsis[J].Plant Science,2012,187:83-88.doi:10.1016/j.plantsci.2012.01.013.
[13] Pandey N,Ranjan A,Pant P,Tripathi R K,Ateek F,Pandey H P,Patre U V,Sawant S V.CAMTA 1 regulates drought responses in Arabidopsis thaliana[J].BioMed Central,2013,14(1):216.doi:10.1186/1471-2164-14-216.
[14] 王芳,王铁兵,李鹏德.外源ABA对干旱胁迫下玉米幼苗氧化损伤的保护作用[J].草业科学,2019,36(11):2887-2894.doi:10.11829/j.issn.1001-0629.2018-0631.
Wang F,Wang T B,Li P D.Protective effects of exogenous ABA on oxidative damage in maize seedlings under drought stress[J].Pratacultural Science,2019,36(11):2887-2894.
[15] Wilkinson S,Kudoyarova G R,Veselov D S,Arkhipova T N,Davies W J.Plant hormone interactions:Innovative targets for crop breeding and management[J].Journal of Experimental Botany,2012,63(9):3499-3509.doi:10.1093/jxb/ers148.
[16] 李保珠,安国勇,韩栓.植物激素ABA在水分胁迫下的功能及信号途径[J].植物生理学报,2012,48(1):11-18.doi:10.13592/j.cnki.ppj.2012.01.012.
Li B Z,An G Y,Han S.Function and signaling of plant hormone ABA under water stress[J].Plant Physiology Communications,2012,48(1):11-18.
[17] 崔维佩,唐桂英,徐平丽,李鹏祥,朱洁琼,单雷.花生种子萌发过程中内源激素含量的变化[J].中国油料作物学报,2020,42(5):869-877.doi:10.19802/j.issn.1007-9084.2019249.
Cui W P,Tang G Y,Xu P L,Li P X,Zhu J Q,Shan L.Content changes of endogenous hormones in peanut seeds during germination[J].Chinese Journal of Oil Crop Sciences,2020,42(5):869-877.
[18] Hu L,Xie Y,Fan S J,Wang Z S,Wang F H,Zhang B,Li H S,Song J,Kong L G.Comparative analysis of root transcriptome profiles between drought-tolerant and susceptible wheat genotypes in response to water stress[J].Plant Science,2018,272:276-293.doi:10.1016/j.plantsci.2018.03.036.
[19] 张小芳,乔潇,俎天娇,张锴,韩金玲,乔亚科,李桂兰.转录组测序筛选野生大豆糖代谢途径干旱相关基因及其表达差异分析[J].中国油料作物学报,2020,42(4):623-631.doi:10.19802/j.issn.1007-9084.2019296.
Zhang X F,Qiao X,Zu T J,Zhang K,Han J L,Qiao Y K,Li G L.Screening and differential expression of drought related genes in carbohydrate metabolism pathway base on transcriptome sequencing in wild soybean[J].Chinese Journal of Oil Crop Sciences,2020,42(4):623-631.
[20] 陈莎莎,兰海燕.植物对盐胁迫响应的信号转导途径[J].植物生理学报,2011,47(2):119-128.doi:10.13592/j.cnki.ppj.2011.02.002.
Chen S S,Lan H Y.Signal transduction pathways in response to salt stress in plants[J].Plant Physiology Communications,2011,47(2):119-128.
[21] 毕影东,刘清醒,郭长虹,郭东林.ABA与植物耐盐信号转导途径的研究进展[J].中国农学通报,2013,29(9):167-171.doi:10.3969/j.issn.1000-6850.2013.09.030.
Bi Y D,Liu Q X,Guo C H,Guo D L.Advances of the ABA and the salt stress tolerance mechanisms in plant signal transduction pathways[J].Chinese Agricultural Science Bulletin,2013,29(9):167-171.
[22] Balo F,Herrera J,Talavera S.Phenotypic consequences of polyploidy and genome size at the microevolutionary scale:a multivariate morphological approach[J].New Phytologist,2011,192(1):256-265.doi:10.1111/j.1469-8137.2011.03787.x.
[23] 宋士伟,焦德志,陈旭,赵泽龙,杨允菲.野大麦对干旱胁迫的生理响应与转录组分析[J].干旱区研究,2019,36(4):909-915.doi:10.13866/j.azr.2019.04.15.
Song S W,Jiao D Z,Chen X,Zhao Z L,Yang Y F.Physiological response and transcriptome of Hordeum brevisubulatum to drought stress[J].Arid Zone Research,2019,36(4):909-915.
[24] 何江峰,王力伟,房永雨,王蕴华,王朝,刘红葵.干旱胁迫和复水处理后梭梭转录因子的转录组分析[J].华北农学报,2020,35(1):36-43.doi:10.7668/hbnxb.20190513.
He J F,Wang L W,Fang Y Y,Wang Y H,Wang C,Liu H K.Transcriptome analysis on transcription factors of Haloxylon ammodendron under drought stress and rehydration treatment[J].Acta Agriculturae Boreali-Sinica,2020,35(1):36-43.
[25] Jeong J S,Kim Y S,Baek K H,Jung H,Ha S H,Do Choi Y,Kim M,Reuzeau C,Kim J K.Root-specific expression of OsNAC10 improves drought tolerance and grain yield in rice under field drought conditions[J].Plant Physiology,2010,153(1):185-197.doi:10.1104/pp.110.154773.
[26] 张然,马祥,朱瑞婷,牛奎举,赵春旭,马晖玲.青海野生草地早熟禾响应干旱胁迫的代谢通路及转录调控分析[J].草地学报,2020,28(6):1508-1518.doi:10.11733/j.issn.1007-0435.2020.06.003.
Zhang R,Ma X,Zhu R T,Niu K J,Zhao C X,Ma H L.Metabolic pathway and transcriptional regulation of Qinghai wild poa pratensis in response to drought stress[J].Acta Agrestia Sinica,2020,28(6):1508-1518.
[27] 王彬,陈敏氡,林亮,叶新如,朱海生,温庆放.植物干旱胁迫的信号通路及相关转录因子研究进展[J].西北植物学报,2020,40(10):1792-1806.doi:10.7606/j.issn.1000-4025.2020.10.1792.
Wang B,Chen M D,Lin L,Ye X R,Zhu H S,Wen Q F.Signal pathways and related transcription factors of drought sress in plants[J].Acta Botanica Boreali-Occidentalia Sinica,2020,40(10):1792-1806.
[28] 高红秀,朱琳,刘天奇,张忠臣.水稻植物激素响应低温胁迫反应的转录组分析[J].分子植物育种,2021,19(13):4188-4197.doi:10.13271/j.mpb.019.004188.
Gao H X,Zhu L,Liu T Q,Zhang Z C.Transcriptomic analysis of plant hormone response to low temperature stress in rice[J].Molecular Plant Breeding,2021,19(13):4188-4197.
[29] Takasaki H,Maruyama K,Kidokoro S,Ito Y,Fujita Y,Shinozaki K,Yamaguchi-Shinozaki K,Nakashima K.The abiotic stress-responsive NAC-type transcription factor OsNAC5 regulates stress-inducible genes and stress tolerance in rice[J].Molecular Genetics and Genomics,2010,284(3):173-183.doi:10.1007/s00438-010-0557-0.
[30] 杨玲,吴玉乾,谢晓东,王根洪,夏庆友.烟草ABF转录因子基因的克隆与生物信息学分析[J].烟草科技,2014(6):73-81,92.doi:10.3969/j.issn.1002-0861.2014.06.016.
Yang L,Wu Y Q,Xie X D,Wang G H,Xia Q Y.Clone and bioinformatics analysis of ABF transcription factor gene from Nicotiana tabacum[J].Tobacco Science & Technology,2014(6):73-81,92.
[31] 张雁明,王莉,张彬,王海岗,彭锁堂,李萍,韩渊怀.谷子ABF3基因对PEG胁迫的响应[J].山西农业大学学报(自然科学版),2013,33(3):191-196.doi:10.3969/j.issn.1671-8151.2013.03.002.
Zhang Y M,Wang L,Zhang B,Wang H G,Peng S T,Li P,Han Y H.The response of ABF3 gene to PEG stress in foxtail millet(Setaria italica L.)[J].Journal of Shanxi Agricultural University (Natural Science Edition),2013,33(3):191-196.
[32] Fujita Y,Yoshida T,Yamaguchi-Shinozaki K.Pivotal role of the AREB/ABF-SnRK2 pathway in ABRE-mediated transcription in response to osmotic stress in plants[J].Physiologia Plantarum,2013,147(1):15-27.doi:10.1111/j.1399-3054.2012.01635.x.
[33] Han S,Lee Y,Park E J,Min M K,Lee Y,Kim T H,Kim B G,Lee S.Structural determinants for pyrabactin recognition in ABA receptors in Oryza sativa[J].Plant Molecular Biology,2019,100(3):319-333.doi:10.1007/s11103-019-00862-6.
[34] Tischer S V,Wunschel C,Papacek M,Kleigrewe K,Hofmann T,Christmann A,Grill E.Combinatorial interaction network of abscisic acid receptors and coreceptors from Arabidopsis thaliana[J].Proceedings of the National Academy of Sciences of the United States of America,2017,114(38):10280-10285.doi:10.1073/pnas.1706593114.
[35] 王华丽,陈宁,杜晗蔚,赵玉龙,杨浩,胡秀丽.高温胁迫下ABA调控sHSP26对玉米叶绿体的保护作用[J].河南农业大学学报,2019,53(6):831-838.doi:10.16445/j.cnki.1000-2340.20191120.015.
Wang H L,Chen N,Du H W,Zhao Y L,Yang H,Hu X L.Function of ABA-regulated sHSP26 in protecting maize(Zea mays L.)chloroplast from heat stress[J].Journal of Henan Agricultural University,2019,53(6):831-838.