鹅是重要水禽资源之一,是肉品、蛋品、羽绒制品和肥肝等产品的重要来源[1],具有重要的经济价值。四川白鹅是肉蛋兼用型优良的地方品种之一,具有肉质营养丰富,生长速度快,繁殖力较高、抗病性强等特点。然而季节性产蛋、就巢性等独特的生理特性,造成鹅产蛋性状不稳定,限制了对鹅产业的进一步发展,目前已有鹅产蛋性状、饲料营养和疫病等重要经济性状的分子标记相关的研究[2-3],但鲜有对鹅产蛋和体组成性状分子标记的研究报道。
促性腺激素抑制激素(Gonadotropin inhibitory hormone,GnIH),重要的下丘脑生殖调控激素之一,是由神经相关肽VF基因(Neuropeptide VF precursor,NPVF)编码的前体蛋白经裂解形成的一种神经十二肽。自首次在鸟类的下丘脑室旁核发现GnIH以来,在哺乳动物、两栖类动物等都发现存在其同源类似物,均作为一种繁殖负调控因子通过其受体(G protein couple receptor 147,GPR147)既可直接作用于垂体抑制促性腺激素的生物合成和释放[4-5],又可通过神经突触投射到正中隆起和GnRH神经元抑制垂体前叶促性腺激素释放[6-8]。研究发现:GnIH激素通过影响血浆中的黄体生成素(Luteinizing hormone,LH)和促卵泡生成素(Follicle-stimulating hormone,FSH)的激素浓度,调控禽类性行为和采食行为等[9-10]。繁殖过程中发挥重要作用外,GnIH 也调控雏鸡和育成期肉种鸡的采食量和青春期发育,外源注射GnIH可提高雏鸡和育成期肉种鸡的采食量,提高体重,促进性成熟[9,11-12]。
GnIH基因碱基突变可引发多种繁殖相关疾病,如人的完全性特发性性腺功能减退症[13]和Kallmann综合征[14]等。前期研究发现,GnIH基因SNPs位点与羊的产羔数显著相关[15]。GnIH的SNP位点与鸡开产日龄、初产蛋质量、开产体质量和300日龄产蛋质量等性状显著或极显著相关[16]。操勇清等[17]发现,GnIH基因C2089T 位点与金定鸭500 日龄产蛋量显著相关。胡彦竞科等[18]研究结果证明,该基因SNP位点与四川白鹅产蛋量显著相关,且鹅的GnIH激素血清浓度和基因表达量在不同繁殖阶段差异显著[19]。因此GnIH的SNP位点可能与动物的繁殖性状相关。
本研究通过克隆鹅GnIH基因DNA序列后,与近源物种进行遗传进化分析。利用PCR产物直接测序的方法,在四川白鹅群体中进行SNP扫描和个体基因型分型,并与体组成和产蛋性状进行关联分析,鉴定鹅的体组成和产蛋性状相关的SNP位点,目的是为上述性状提供潜在的分子标记。
1 材料和方法
1.1 试验动物和数据采集
选择重庆市荣昌区安富水禽实验基地饲养的同一批次孵化的208只四川白鹅(母鹅)为试验材料。为准确记录性状,鹅孵化出壳后即记录鹅出生质量,并进行系统的脚号和翅号标记。饲养至28周龄,转移到个体笼(600 mm×800 mm×900 mm)中继续饲养,鹅群在个体笼中饲养至65周(休产期),期间自由采食和饮水。转移前进行翅静脉血收集,利用血液DNA提取试剂盒(北京天根,DP332)抽提鹅血液基因组DNA,并将其溶解在TE溶液中在-20 ℃保存备用。每天进行2次产蛋数记录,最后统计初产蛋质量、48周产蛋数、64周产蛋数;鹅群转移出个体笼后,根据体组成性状的测量方法[20],利用软尺测量每只鹅的成年颈长、胸宽、半潜水长、骨盆宽、体斜长、胫长、龙骨长和胫围等体组成性状。
1.2 GnIH基因克隆、SNP多态性位点检测和分型与遗传进化分析
本研究以四川白鹅的基因组DNA为模板,设计合适的PCR引物(表1),直接测序法获得GnIH基因序列、SNP多态性位点和基因分型结果。扩增体系50 μL:基因组DNA(<1 μg) 1 μL,上、下游引物(10 μmol/L)各1 μL,2×Gflex PCR Buffer 25 μL,Tks Gflex DNA 聚合酶1 μL,ddH2O2 1 μL。PCR扩增程序:98 ℃预变性2 min;98 ℃变性30 s,退火30 s,72 ℃延伸10 s,30个循环;72 ℃延伸10 min。PCR产物鉴定后由Invitrogen公司测序,用DNAStar软件对测序结果序列进行比对分析和SNP扫描,获得的序列与其他物种的GnIH基因采用Mega 6.0软件的邻接法建树,分析遗传进化关系。参照命名系统(www.hgvs.org/mutnomen)对多态位点进行统一命名。
表1 四川白鹅GnIH基因引物序列
Tab.1 The primer sequences for GnIH sequence cloning in Sichuan white goose

引物名称Primer引物序列(5′-3′)Primer sequence扩增长度/bpPCR size退火温度/℃Tm试验目的Experimental designGnIH1FAGATTCTGAGCTTTCTAACT2 32058.0克隆GnIH1RAATACTTGTTTATTGTTACCTGnIH2FAAGGTATGCAGGACAACGTCGTC1 88361.0克隆GnIH2R TTCACACAGTAGGATTCTTTTTATTGnIH-SNP-F40TGGAAGTCATTTCAACCCAGAAG1 70160.0SNP扫描GnIH-SNP-R1741CGACGTTGTCCTGCATACCTGnIH-SNP-F1532CCAGCATCTTCTCCCCAAGA1 51358.0SNP扫描GnIH-SNP-R3045TTAGCCAACTGAAACGTGGTGnIH-SNP-F2987ACAGTCCTTCTTTTCATAGCTGC74856.4克隆/SNP扫描GnIH-SNP-R3735TCTTGCGGGACTCATAAACAAC
1.3 统计分析
利用单因素方差分析模型分析GnIH基因SNP多态性位点及其构建的单倍型与性状之间的关联性,模型:Y=μ+Gi+eij,其中Y为性状观测值,μ是群体平均值,Gi为个体基因型,eij为随机误差。利用JMP(version 13.0)软件计算各个性状对应的基因型的最小二乘均数。显著差异水平为P <0.05,极显著差异水平为P <0.01。
2 结果与分析
2.1 GnIH基因克隆和遗传进化分析结果
PCR克隆获得了四川白鹅GnIH基因3 735 bp(图1-A),该序列包含3个外显子和2个内含子,并含有完整的CDS区长522 bp,编码一个含173个氨基酸的前体蛋白,蛋白包括1个具有生物活性的十二肽和2个GnIH相关肽(GnIH-RP1和GnIH-RP2),其中活性十二肽GnIH氨基酸序列为:SIKPIANLPLRF(图1-B)所示。获得的序列上传NCBI,序列号T862215。Mega 6.0对GnIH基因进行遗传进化分析,结果显示,四川白鹅与绿头鸭的GnIH序列的相似性最高,达到99%,鸟类与哺乳类动物的GnIH处在进化树上的2个大的分支上(图1-C)。
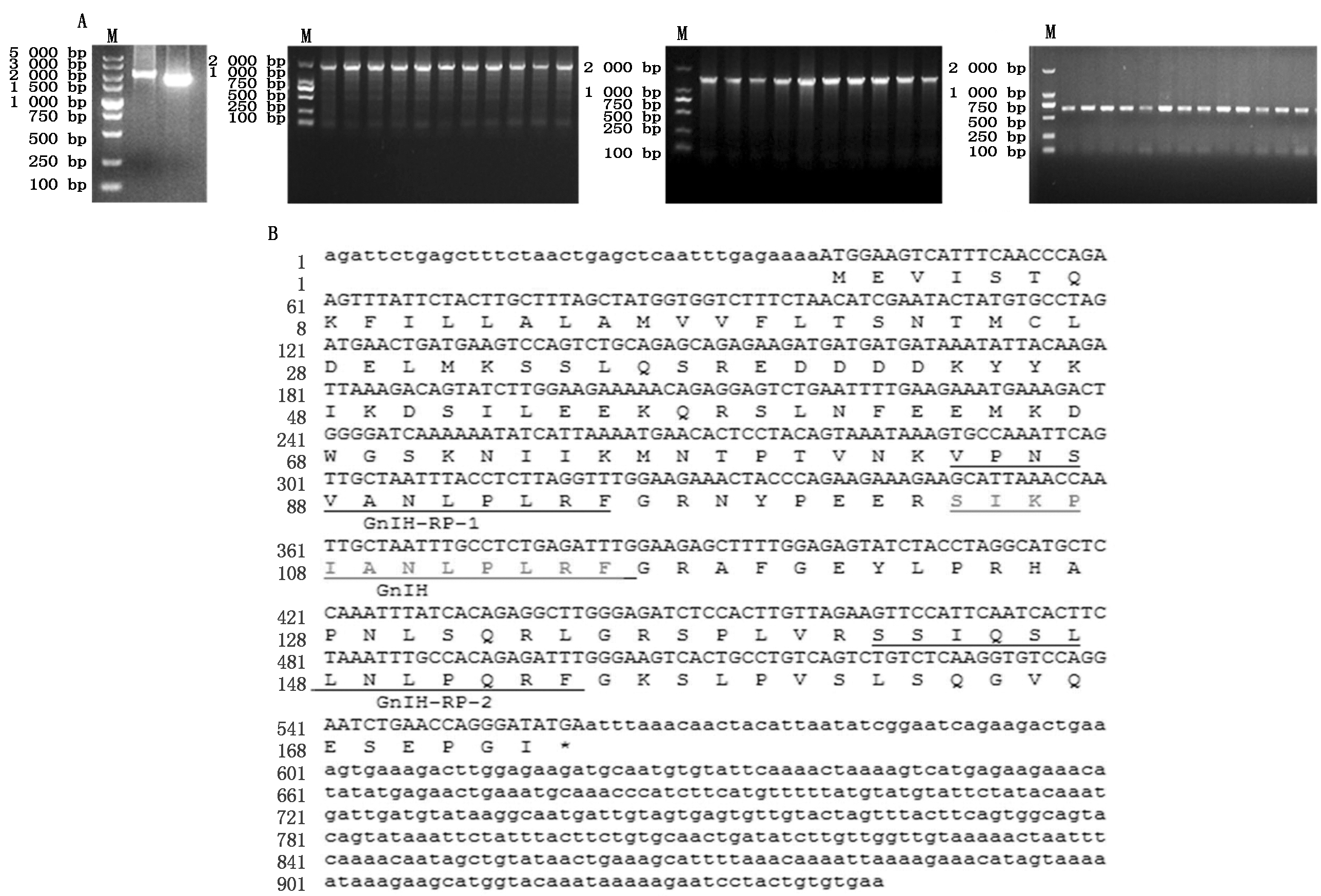
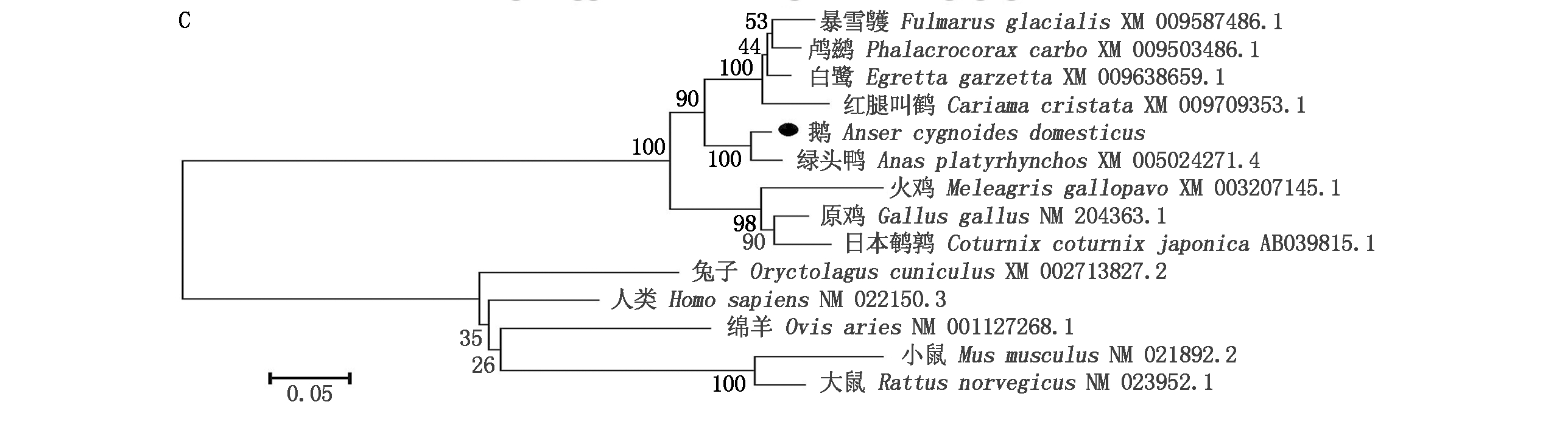
A.GnIH基因克隆和SNP扫描引物PCR扩增结果; B.GnIH(NPVF)mRNA编码前体肽结构; C.四川白鹅GnIH(NPVF)基因mRNA与其他物种GnIH(NPVF)基因mRNA序列相似性比较分析。M.DNA Marker;下划线标示的氨基酸序列分别代表前体肽裂解后形成的GnIH相关肽1、GnIH活性十二肽和GnIH相关肽2,其中红色标出的氨基酸序列代表GnIH活性十二肽。
A.PCR amplification results by the primers for cloning and analyzing polymorphism of the GnIH gene;B.The precursor peptide structure of the GnIH(NPVF)gene mRNA coded;C.Comparative analysis of sequence similarity of GnIH(NPVF)gene mRNA in the Sichuan white goose with other species.M.DNA Marker;The underlined amino acid sequence represented the GnIH-related peptide 1,GnIH active dodecapeptide and GnIH-related peptide 2 cloven by the precursor peptide,among which the red marked amino acid sequence represented GnIH active dodecapeptide.
图1 四川白鹅GnIH(NPVF)基因扩增与序列分析
Fig.1 Cloning and sequential analysis of GnIH in Sichuan white goose
2.2 GnIH基因SNP与四川白鹅产蛋和体组成性状的相关分析
本研究发现26个SNP位点在四川白鹅群体中具有多态性,g.2164C>G位点突变导致脯氨酸变成精氨酸(P>R),g.2228T>A位点突变导致酪氨酸变成天冬氨酸(Y>D)(表2)。GnIH基因的多态性位点与四川白鹅产蛋和体组成性状的相关分析结果表明(表2):初产蛋质量与g.811T>C和g.1892T>C位点显著相关(P<0.05)与g.1320G>A位点极显著相关(P<0.01);体斜长与g.1809G>T位点显著相关(P<0.05);颈长与g.2164C>G位点极显著相关(P<0.01);胸宽与g.1708A>G和g.2164C>G位点显著相关(P<0.05);胫围与g.1809G>T、g.1892T>C、g.1929C>A和g.2164C>G位点显著或极显著相关(P<0.05或P<0.01),然而与48周产蛋数、64周产蛋数、出生质量、半潜水长、龙骨长、骨盆宽等无显著相关(P> 0.05)。
表2 GnIH突变位点对四川白鹅产蛋性状和体组成性状的影响(P值)
Tab.2 The effect of GnIH mutations on egg weight and body measurement traits in Sichuan white goose
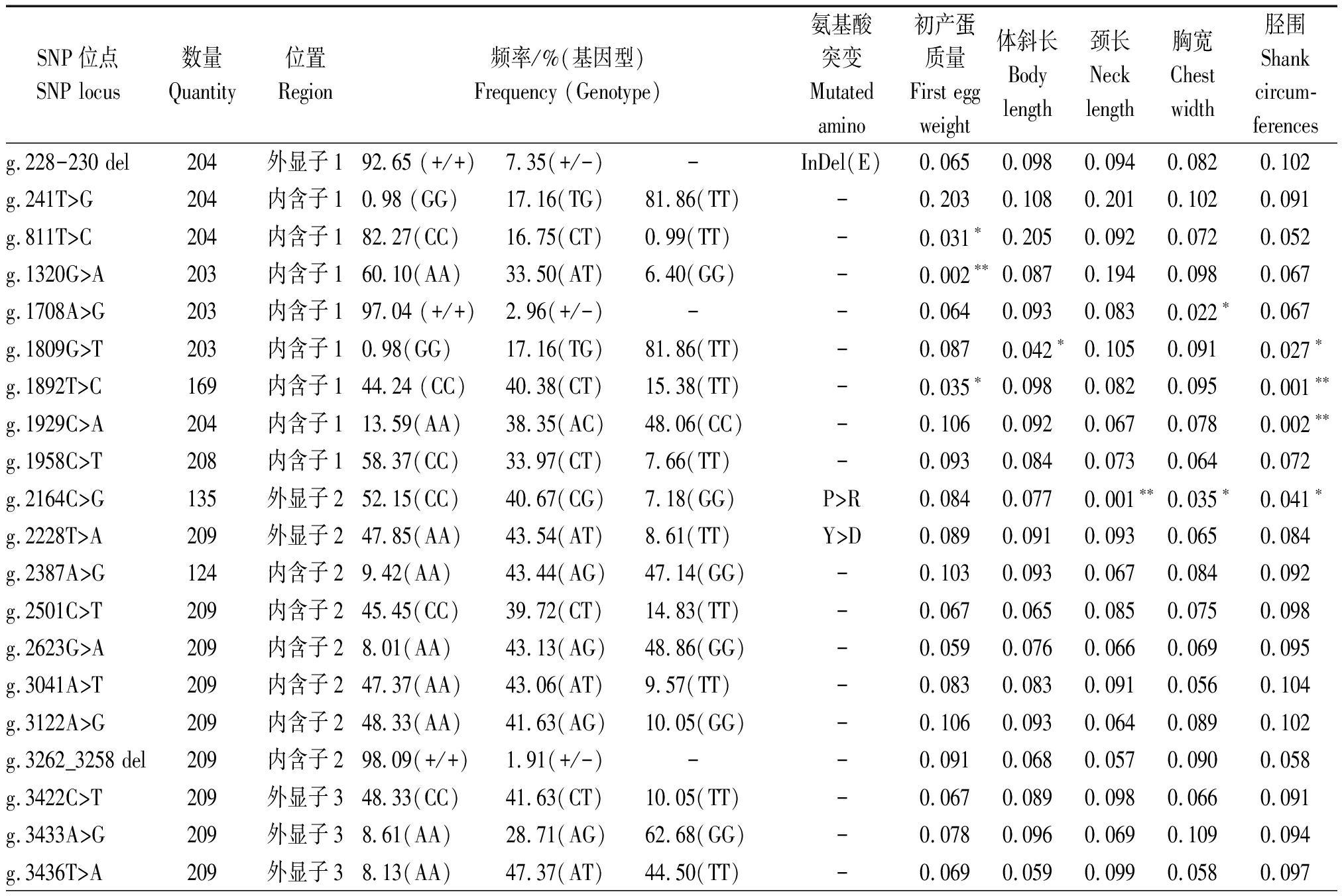
SNP位点SNP locus数量Quantity位置Region频率/%(基因型)Frequency (Genotype)氨基酸突变Mutatedamino初产蛋质量First egg weight体斜长Body length颈长Neck length胸宽Chest width胫围 Shank circum-ferences g.228-230 del204外显子192.65 (+/+)7.35(+/-) -InDel(E)0.0650.0980.0940.0820.102g.241T>G204内含子10.98 (GG)17.16(TG)81.86(TT)-0.2030.1080.2010.1020.091g.811T>C204内含子182.27(CC)16.75(CT)0.99(TT)-0.031∗0.2050.0920.0720.052g.1320G>A203内含子160.10(AA)33.50(AT)6.40(GG)-0.002∗∗0.0870.1940.0980.067g.1708A>G203内含子197.04 (+/+)2.96(+/-) --0.0640.0930.0830.022∗0.067g.1809G>T203内含子10.98(GG)17.16(TG)81.86(TT)-0.0870.042∗0.1050.0910.027∗g.1892T>C169内含子144.24 (CC)40.38(CT)15.38(TT)-0.035∗0.0980.0820.0950.001∗∗g.1929C>A204内含子113.59(AA)38.35(AC)48.06(CC)-0.1060.0920.0670.0780.002∗∗g.1958C>T208内含子158.37(CC)33.97(CT)7.66(TT)-0.0930.0840.0730.0640.072g.2164C>G135外显子252.15(CC)40.67(CG)7.18(GG)P>R0.0840.0770.001∗∗0.035∗0.041∗ g.2228T>A209外显子247.85(AA)43.54(AT)8.61(TT)Y>D0.0890.0910.0930.0650.084g.2387A>G124内含子29.42(AA)43.44(AG)47.14(GG)-0.1030.0930.0670.0840.092g.2501C>T209内含子245.45(CC)39.72(CT)14.83(TT)-0.0670.0650.0850.0750.098g.2623G>A209内含子28.01(AA)43.13(AG)48.86(GG)-0.0590.0760.0660.0690.095g.3041A>T209内含子247.37(AA)43.06(AT)9.57(TT)-0.0830.0830.0910.0560.104g.3122A>G209内含子248.33(AA)41.63(AG)10.05(GG)-0.1060.0930.0640.0890.102g.3262_3258 del209内含子298.09(+/+)1.91(+/-) --0.0910.0680.0570.0900.058g.3422C>T209外显子348.33(CC)41.63(CT)10.05(TT)-0.0670.0890.0980.0660.091g.3433A>G209外显子38.61(AA)28.71(AG)62.68(GG)-0.0780.0960.0690.1090.094g.3436T>A209外显子38.13(AA)47.37(AT)44.50(TT)-0.0690.0590.0990.0580.097
表2(续)
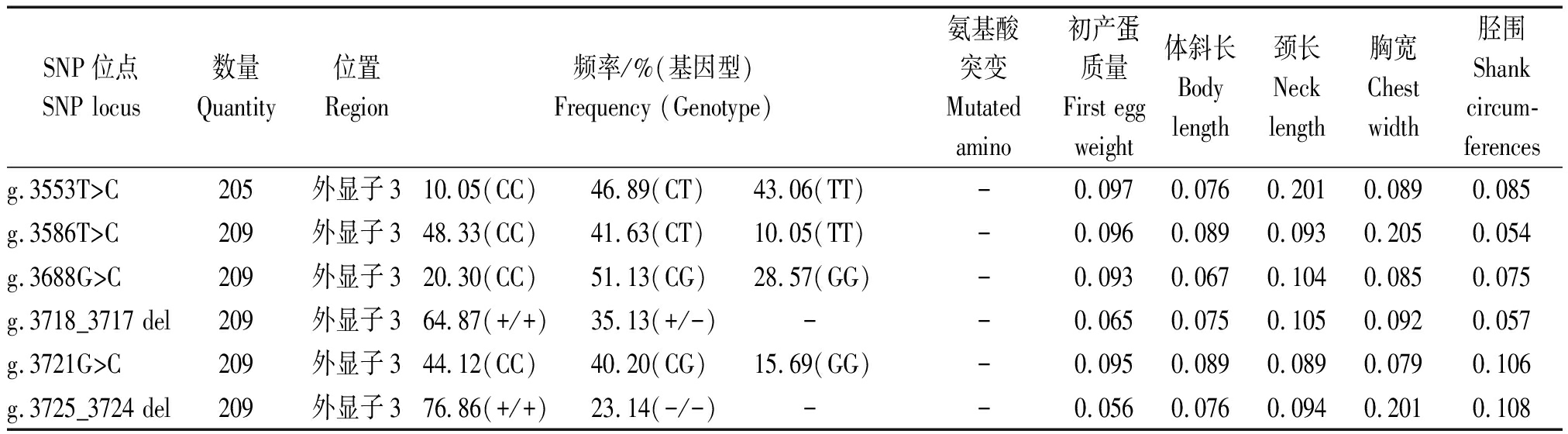
SNP位点SNP locus数量Quantity位置Region频率/%(基因型)Frequency (Genotype)氨基酸突变Mutatedamino初产蛋质量First egg weight体斜长Body length颈长Neck length胸宽Chest width胫围 Shank circum-ferences g.3553T>C205外显子310.05(CC)46.89(CT)43.06(TT)-0.0970.0760.2010.0890.085g.3586T>C209外显子348.33(CC)41.63(CT)10.05(TT)-0.0960.0890.0930.2050.054g.3688G>C209外显子320.30(CC)51.13(CG)28.57(GG)-0.0930.0670.1040.0850.075g.3718_3717 del209外显子364.87(+/+)35.13(+/-) --0.0650.0750.1050.0920.057g.3721G>C209外显子344.12(CC)40.20(CG)15.69(GG)-0.0950.0890.0890.0790.106g.3725_3724 del209外显子376.86(+/+)23.14(-/-) --0.0560.0760.0940.2010.108
注:*.P值差异显著(P<0.05);**.P值差异极显著(P<0.01)。
Note:*.There was significant difference(P<0.05);**.There was extremely significant difference(P<0.01).
SNP位点基因型多重比对结果表明:g.811T>C、g.1320G>A和g.1892T>C的CC、GG和CC基因型的个体的初产蛋质量均极显著高于其他基因型个体(P<0.01)(表3);g.1809G>T的GG基因型个体的体斜长极显著高于TT基因型个体(P<0.01);g.2164C>G位点GG基因型个体的颈长显著低于CC基因型个体(P<0.05);g.1708A>G位点的GG基因型个体的胸宽显著高于AG和AA基因型个体,而g.2164C>G位点的AA基因型个体的胸宽极显著高于其他基因型(P< 0.01);对于胫围性状,g.1809G>T 的GT性状显著低于其他基因型(P< 0.05),g.1809G>T、g.1892T>C、g.1929C>A和g.2164C>G位点的GG、TT、AA和CC基因型个体的胫围极显著高于其他基因型(P <0.01)(表3)。
表3 GnIH 不同基因型对鹅产蛋和体组成性状的影响
Tab.3 The effect of genotypes of GnIH on the egg-laying
traits and body measurement traits
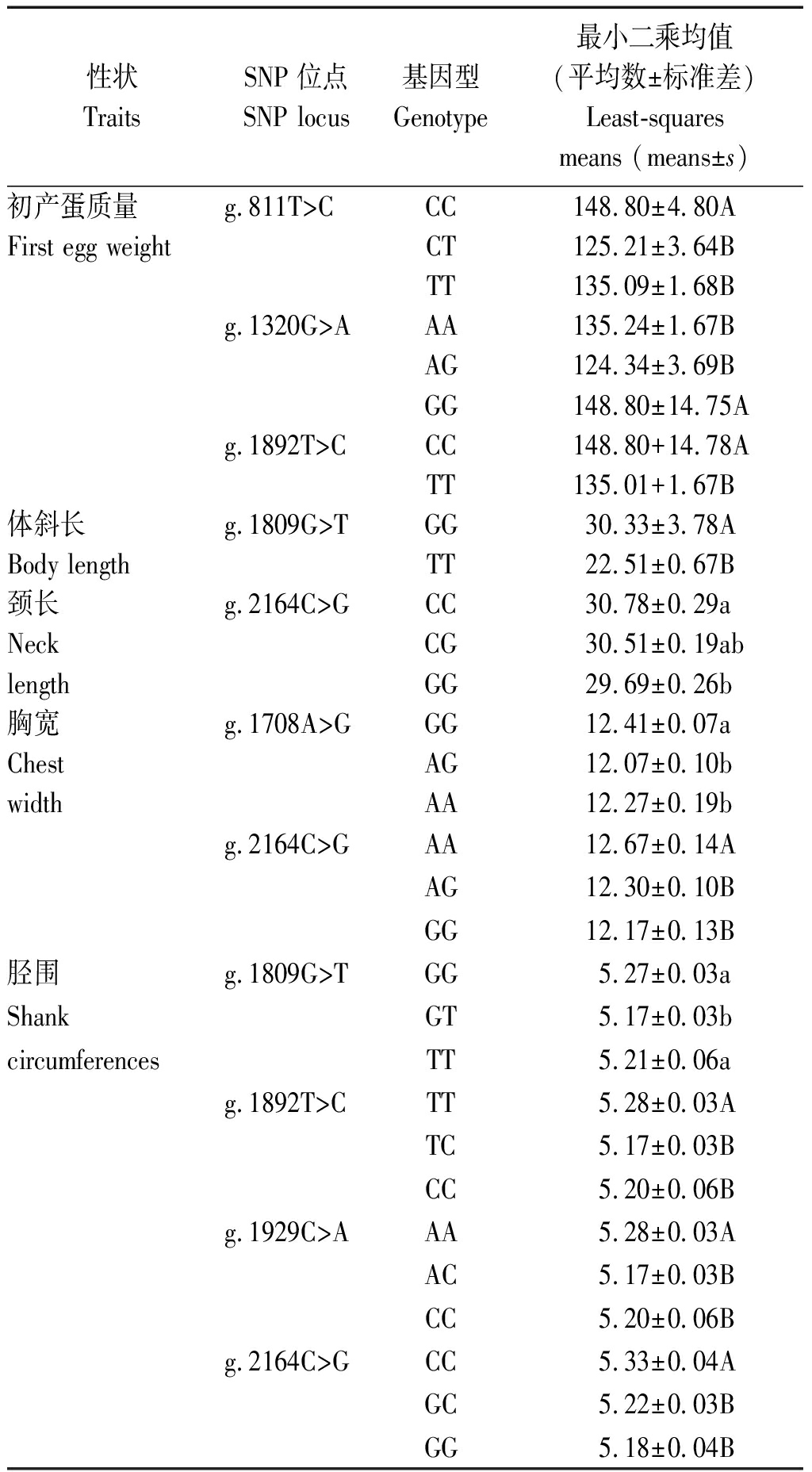
性状TraitsSNP位点SNP locus基因型Genotype最小二乘均值(平均数±标准差)Least-squares means (means±s)初产蛋质量g.811T>CCC148.80±4.80AFirst egg weightCT125.21±3.64BTT135.09±1.68Bg.1320G>AAA135.24±1.67BAG124.34±3.69BGG148.80±14.75Ag.1892T>CCC148.80+14.78ATT135.01+1.67B体斜长g.1809G>TGG30.33±3.78ABody lengthTT22.51±0.67B颈长g.2164C>GCC30.78±0.29aNeckCG30.51±0.19ablengthGG29.69±0.26b胸宽g.1708A>GGG12.41±0.07aChestAG12.07±0.10bwidthAA12.27±0.19bg.2164C>GAA12.67±0.14AAG12.30±0.10BGG12.17±0.13B胫围g.1809G>TGG5.27±0.03aShankGT5.17±0.03bcircumferencesTT5.21±0.06ag.1892T>CTT5.28±0.03ATC5.17±0.03BCC5.20±0.06Bg.1929C>AAA5.28±0.03AAC5.17±0.03BCC5.20±0.06Bg.2164C>GCC5.33±0.04AGC5.22±0.03BGG5.18±0.04B
注:不同小写字母表示差异显著(P<0.05);不同大写字母表示差异极显著(P<0.01)。
Note:The different lowercase letters represented the significant difference between genotypes (P<0.05);The different uppercase letters represented the extremely significant difference between genotypes (P<0.01).
3 讨论与结论
3.1 关于四川白鹅GnIH基因序列结构的讨论
本研究发现:GnIH成熟十二肽(SIKPIANLPLRF)及其前体蛋白上的2个相关肽GnIH-RP-1 和 GnIH-RP-2都拥有保守的C端LPX(L/Q)RF结构,这个保守基序在其他脊柱动物也被发现,如RFRP1、RFRP3等[21-22];并发现鹅GnIH基因与绿头鸭的GnIH遗传进化关系最近,相似性达到99%,鸭和鹅都属于鸭科动物,因此鹅和鸭亲缘关系最近,这也解释鹅GnIH与其他禽类或鸟类聚在一个大的分支上。
研究表明,GnIH基因在鸟类、灵长类或两栖类动物中,其功能都是保守的,通过调控激素分析调节动物繁殖[23-24]。Ubuka等[25]研究了最古老的脊椎动物无颌类GnIH前体基因,免疫组化结果显示,GnIH神经纤维与GnRH3神经元胞体接触,影响GHTβ的表达,虽然拥有共同的始祖基因,但在进化过程哺乳类和鸟类GnIH处在进化树上2个大的分支上,其可能是因为直系或旁系遗传的差异导致[26]。
3.2 关于四川白鹅GnIH基因多肽性与繁殖性状关系的讨论
鹅作为一种短日照光周期家禽,在周期性繁殖、繁殖行为调控中均扮演重要作用[11]。前期研究表明,GnIH在鹅的不同的繁殖阶段HPO轴中显著性差异表达,进一步佐证GnIH参与了鹅的周期性繁殖调控[19]。初产蛋质量是鹅重要的经济指标之一,本研究发现GnIH基因内含子1上多个SNP位点(g.811T>C、 g.1320G>A和g.1892T>C)与初产蛋质量显著或极显著相关(P <0.05或 P <0.01)。GnIH激素能在下丘脑水平同抑制促卵泡生成素和促黄体素的分泌,间接参与卵巢卵泡的发育成熟及排卵。除了下丘脑水平的抑制繁殖作用外,GnIH受体也在卵泡颗粒层细胞表达,直接参与卵泡的生长、成熟。此外,猫的腔前卵泡中发现GnIH的同源类似物RFRP3促进卵泡基底膜降解、细胞凋亡并抑制孕酮分泌,最终导致卵泡闭锁[27]。卵泡的发育结果最终反映在蛋的品质、数量及质量上。由此推测,GnIH基因上的碱基突变可能会影响卵泡生产成熟、最终影响初产蛋质量性状。
3.3 关于四川白鹅GnIH基因多肽性与体尺性状关系的讨论
基因外显子2上的SNP位点g.2164C>G与体尺性状的多个指标,如颈长、胸宽和胫围均出现显著性相关。基因上的这个突变位点导致外显子2上编码的脯氨酸突变为精氨酸,位于成熟肽裂解位点(PEER)旁,这一突变潜在影响GnIH成熟肽的生成。Lima等[28]研究人的GnIH编码基因NPVF及其受体GPR147基因多态性与2种促性腺激素释放激素依赖性青春期疾病(特发性中枢性早熟和常压下孤立性促性腺激素低下症)的发生的关联性分析,发现GnIH前体蛋白71位异亮氨酸的缺失与特发性性早熟显著相关。目前,尚没有鹅GnIH与体组成性状关联的相关文献报道,但已有研究表明GnIH在摄食、生长及糖代谢等方面发挥重要作用[29],这些环节均会间接影响鹅的体组成性状。Huo等[30]通过腹腔注射GnIH同源类似物RFRP3来观测其对大鼠摄食量、膳食结构、体质量和葡萄糖代谢的影响。结果表明,大鼠腹腔注射RFRP3后,大鼠体质量明显增加,高吞噬、高脂血症、高血糖、糖耐量低、胰岛素低、高血糖、胰岛素抵抗,胰岛大小和炎症反应显著增加,推测RFRP3可作为一种新的神经内分泌调节因子参与血糖稳态。Gotlieb等[31]研究证实了在排除卵巢E2负反馈调节的情况下,RFRP3不是通过mPOAGnRH或RFRP3受体GPR147mrna来抑制循环LH排卵峰,而是通过抑制弓状核Kiss1mRNA表达,进而直接影响垂体性腺激素。由此推测,作为繁殖调控的重要基因GnIH基因能从采食、性激素调控、血糖调节等多个环节参与到鹅生长发育过程,影响鹅的体组成性状。
GnIH基因的SNP位点与初产蛋质量、体斜长、胫长、胸宽、胫围等性状显著或极显著相关(P<0.05或P<0.01),而与成48周龄蛋数、64周龄蛋数、出生体质量、半潜水长、骨盆宽、龙骨长等性状相关性不显著(P>0.05)。GnIH基因的SNP位点可作为初产蛋质量、体斜长、胫长、胸宽、胫围等性状的潜在的分子标记。
[1] Goluch-Koniuszy Z,Haraf G. Geese for slaughter and wild geese as a source of selected mineral elements in a diet[J].Journal of Elementology,2018,23(4): 1343-1360. doi:10.5601/jelem.2018.23.2.1594.
[2] Wang Z,Zhao J. Pathogenesis of hypervirulent fowl adenovirus serotype 4:the contributions of viral and host factors [J].Viruses,2019,11(8):741.doi: 10.3390/v11080741.
[3] Ying S J,Qin J L,Dai Z C,An H,Zhu H X,Chen R,Yang X J,Wu W D,Shi Z D. Effects of LPS on the secretion of gonadotrophin hormones and expression of genes in the Hypothalamus-Pituitary-Ovary(HPG)axis in laying Yangzhou geese[J].Animals,2020,10(12):2259.doi:10.3390/ani10122259.
[4] Tsutsui K,Saigoh E,Ukena K,Teranishi H,Fujisawa Y,Kikuchi M,Ishii S,Sharp P J. A novel avian hypothalamic peptide inhibiting gonadotropin release[J].Biochemical and Biophysical Research Communications,2000,275(2):661-667. doi:10.1006/bbrc.2000.3350.
[5] Kriegsfeld L J,Jennings K J,Bentley G E,Tsutsui K. Gonadotrophin-inhibitory hormone and its mammalian orthologue RFamide-related peptide-3:Discovery and functional implications for reproduction and stress[J].Journal of Neuroendocrinology,2018,30(7):e12597. doi:10.1111/jne.12597.
[6] Jadhao A G,Pinelli C,D′ Aniello B,Tsutsui K. Gonadotropin-inhibitory hormone(GnIH)in the amphibian brain and its relationship with the gonadotropin releasing hormone(GnRH)system:An overview[J].General and Comparative Endocrinology,2017,240:69-76. doi:10.1016/j.ygcen. 2016.09.006.
[7] Kiyohara M,Son Y L,Tsutsui K. Involvement of gonadotropin-inhibitory hormone in pubertal disorders induced by thyroid status[J]. Scientific Reports,2017,7(1):1042.doi:10.1038/s41598-017-01183-8.
[8] Kriegsfeld L J,Mei D F,Bentley G E,Ubuka T,Mason A O,Inoue K,Ukena K,Tsutsui K,Silver R. Identification and characterization of a gonadotropin-inhibitory system in the brains of mammals[J].Proc Natl Acad Sci U S A,2006,103(7):2410-2415. doi:10.1073/pnas.0511003103.
[9] Tachibana T,Sato M,Takahashi H,Ukena K,Tsutsui K,Furuse M. Gonadotropin-inhibiting hormone stimulates feeding behavior in chicks[J].Brain Research,2005,1050(1/2):94-100. doi:10.1016/j.brainres.2005.05.035.
[10] Ubuka T,Parhar I,Kriegsfeld L J,Tsutsui K. Editorial:The roles of GnIH in reproductive function and behavior[J].Frontiers in Endocrinology,2018,9:19. doi:10.3389/fendo.2018.00019.
[11] Tsutsui K,Ubuka T. GnIH control of feeding and reproductive behaviors[J].Frontiers in Endocrinology,2016,7:170. doi:10.3389/fendo.2016.00170.
[12] Hadinia S H,Carneiro P R O,Fitzsimmons C J,B d carrats G Y,Zuidhof M J. Post-photostimulation energy intake accelerated pubertal development in broiler breeder pullets[J].Poultry Science,2020,99(4):2215-2229.doi:10.1016/j.psj.2019.11.065.
[13] Mengen E,Tunc S,Kotan L D,Nalbantoglu O,Demir K,Curbuz F,Turan I,Seker G,Yuksel B,Topaloglu A K. Complete idiopathic hypogonadotropic hypogonadism due to homozygous GNRH1 mutations in the mutational hot spots in the region encoding the decapeptide[J].Hormone research in paediatrics,2016,85(2):107-111.doi:10.1159/000441977.
[14] Stamou M I,Georgopoulos N A. Kallmann syndrome:Phenotype and genotype of hypogonadotropic hypogonadism[J].Metabolism:Clinical and Experimental,2018,86:124-134.doi:10.1016/j.metabol.2017.10.012.
[15] 严妍.山羊GnIH基因SNP的研究及其与产羔数的关联分析[D].杨凌:西北农林科技大学,2013.
Yan Y. Relationship of SNP of GnIH with prolific performence of goats[D].Yangling:Northwest A & F University,2013.
[16] 黄钦柯.地方蛋鸡新品系基础群产蛋性能及相关候选基因(GnIH)多态性的研究[D].雅安:四川农业大学,2013.
Huang Q K. Studies on egg-laying performance and polymorphism of relavent candidate genes(GnIH)in the underlying group of new local hens[D].Yaan:Sichuan Agricultural University,2013.
[17] 操勇清,曾涛,刘国发,周玮,石放雄,卢立志. 金定鸭卵抑制剂基因(OIH)和促性腺激素抑制激素基因(GnIH)多态性与产蛋性能的相关性研究[J].农业生物技术学报,2020,28(2):282-290.doi:10.3969/j.issn.1674-7968.2020.02.010.
Cao Y Q,Zeng T,Liu G F,Zhou W,Shi F X,Lu L Z. Correlation study on polymorphism of ovoinhibitor(OIH)and gonadotropin-inhibitor hormone(GnIH)with laying performance in Jinding Duck(Anas platyrhynchos domestica)[J].Journal of Agricultural Biotechnology,2020,28(2):282-290.
[18] 胡彦竞科,张克山,钟航,王启贵,刘安芳. 鹅GnIH基因多态性及其与产蛋量的关联性研究[J].中国畜牧杂志,2016,52(21):17-19,104.doi:10.3969/j.issn.0258-7033.2016.21.005.
Hu Y J K,Zhang K S,Zhong H,Wang Q G,Liu A F. Correlation study on polymorphism of gonadotropin-inhibitor hormone(GnIH)with laying performance in goose[J].Chinese Journal of Animal Science,2016,52(21):17-19,104.
[19] 张克山,胡彦竞科,韩笑哲,高广亮,钟航,王启贵.鹅不同繁殖时期GnRH和GnIH基因表达和激素浓度变化分析[J].畜牧兽医学报,2016,47(8):1720-1726.doi:10.11843/j.issn.0366-6964.2016.08.025.
Zhang K S,Hu Y J K,Han X Z,Gao G L,Zhong H,Wang Q G.Analysis of the serum concentrations and mRNA expression levels of GnRH and GnIH in geese during different reproductive periods[J].Acta Veterinaria et Zootechnica Sinica,2016,47(8):1720-1726.
[20] 李欣钰,邱晓辉,陈昌义,俞钦明,刘六香,兰旅涛. 广丰白翎鹅体重与体尺性状指标主成分分析[J].中国畜牧兽医,2012,39(9):164-168.doi:10.3969/j.issn.1671-7236.2012.09.041.
Li X Y,Qiu X H,Chen C Y,Yu Q M,Liu L X,Lan L T. Principal component analysis of body weight and body measurement of Guangfeng white geese[J].China Animal Husbandry & Veterinary Medicine,2012,39(9):164-168.
[21] Tsutsui K,Osugi T,Son Y L,Ubuka T. Review:Structure,function and evolution of GnIH[J].General and Comparative Endocrinology,2018,264:48-57.doi:10.1016/j.ygcen.2017.07.024.
[22] Ubuka T,Tsutsui K. Reproductive neuroendocrinology of mammalian gonadotropin-inhibitory hormone[J].Reproductive Medicine and Biology,2019,18(3):225-233.doi:10.1002/rmb2.12272.
[23] Tsutsui K,Bentley G E,Bedecarrats G,Osugi T,Ubuka T,Kriegsfeld L J. Gonadotropin-inhibitory hormone(GnIH)and its control of central and peripheral reproductive function [J].Frontiers in Neuroendocrinology,2010,31(3):284-295.doi:10.1016/j.yfrne.2010.03.001.
[24] Ubuka T,Son Y L,Tsutsui K. Molecular,cellular,morphological,physiological and behavioral aspects of gonadotropin-inhibitory hormone[J].General and Comparative Endocrinology,2016,227:27-50.doi:10.1016/j.ygcen.2015.09.009.
[25] Ubuka T,Tsutsui K. Evolution of gonadotropin-inhibitory hormone receptor and its ligand[J].General and Comparative Endocrinology,2014,209:148-161.doi:10.1016/j.ygcen.2014.09.002.
[26] Lederberg J. Linear inheritance in transductional clones[J].Genetics,1956,41(6):845-871.doi:10.1093/genetics/41.6.845.
[27] Wilsterman K,Bentley G E,Comizzoli P. RFRP3 influences basal lamina degradation,cellular death,and progesterone secretion in cultured preantral ovarian follicles from the domestic cat[J].Peer J,2019,7:e7540. doi:10.7717/peerj.7540.
[28] Lima C J,Cardoso S C,Lemos E F,Zingler E,Capanema C,Menezes L D,Vogado G,Dos Santos B T,de Moraes O L,Duarte E F,de Brito V N,Latronico A C,Lofrano-Porto A. Mutational analysis of the genes encoding RFamide-related peptide-3,the human orthologue of gonadotrophin-inhibitory hormone,and its receptor(GPR147)in patients with gonadotrophin-releasing hormone-dependent pubertal disorders[J].Journal of Neuroendocrinology,2014,26(11):817-824. doi:10.1111/jne.12207.
[29] McConn B R,Yi J Q,Gilbert E R,Siegel P B,Chowdhury V S,Furuse M,Cline M A. Stimulation of food intake after central administration of gonadotropin-inhibitory hormone is similar in genetically selected low and high body weight lines of chickens[J].General and Comparative Endocrinology,2016,232:96-100.doi:10.1016/j.ygcen.2016.01.004.
[30] Huo K L,Li X,Hu W,Song X X,Zhang D N,Zhang X,Chen X Y,Yuan J Z,Zuo J Y,Wang X Y. RFRP-3,the mammalian ortholog of GnIH,is a novel modulator involved in food intake and glucose homeostasis[J].Frontiers in Endocrinology,2020,11:1-15.doi:10.3389/fendo.2020.00194.
[31] Gotlieb N,Baker C N,Moeller J,Kriegsfeld L J. Time-of-day-dependent sensitivity of the reproductive axis to RFamide-related peptide-3 inhibition in female Syrian hamsters[J].Journal of Neuroendocrinology,2019,31(11):e12798.doi:10.1111/jne.12798.