马铃薯是我国第四大主粮作物,2019年中国马铃薯种植面积478.95万hm2,总产量9 193.8万t,种植面积和总产量均占世界第一位[1]。
马铃薯生长过程中会遭受病毒的威胁,导致马铃薯种性退化、产量下降以及品质变劣。马铃薯X病毒(Potato virus X,PVX)是传播范围最广的马铃薯病毒[2],马铃薯Y病毒(Potato virus Y,PVY)是对马铃薯危害最为严重的病毒[3-4],马铃薯纺锤块茎类病毒(Potato spindle tuber viroid,PSTVd)可造成马铃薯块茎裂口且不能通过茎尖脱毒汰除[5]。当2种或者2种以上病毒复合侵染马铃薯植株时,带来的损失要远高于单一病毒侵染[6-7]。
转化病毒外壳蛋白基因而获得抗病毒植株是植物抗病毒基因工程的重要方法[8]。利用RNA干扰技术将病毒来源的基因转化植物获得抗性植株,可提高植物对病毒抗性,且此法安全有效[9]。amiRNA技术是利用植物体内固有的通过miRNA进行基因沉默的系统,以拟南芥miR159a等前体序列作为amiRNA的基木骨架序列,通过人工设计特异的miRNA,经重叠PCR扩增将天然miRNA的成熟序列替换成amiRNA的成熟序列来沉默目的基因的方法[10]。amiRNA技术具有高沉默效率、稳定遗传、高特异性等优点[11]。利用amiRNA技术培育抗病毒植物已有大量报道[12-15],然而关于利用amiRNA策略培育多抗病毒的转基因植株方面的报道还较少。
本研究以拟南芥的pre-miRl59a为骨架,分别针对PVX编码的P25、PVY编码的HC-Pro以及PSTVd编码的Vip1基因设计amiRNA并构建表达载体,利用农杆菌介导法转化马铃薯品种费乌瑞它转基因植株并对T0转基因植株进行病毒抗性鉴定,amiRNA定量分析等。本研究利用miRNA介导兼抗PVX、PVY和PSTVd马铃薯植株为实现马铃薯兼抗多种病毒提供了新途径,也为获得马铃薯抗病毒病提供理论依据和基因资源。
1 材料和方法
1.1 试验材料
植物材料:以马铃薯品种费乌瑞它为植物材料,费乌瑞它脱毒试管苗由黑龙江八一农垦大学马铃薯研究所保存。费乌瑞它是早熟鲜食型品种,中抗PVY,但在PVX和PVY混合侵染下,表现为重花叶、植株皱缩严重,产量下降45.7%[16];费乌瑞它感染PSTVd时块茎变细长、呈现豌豆状,部分芽眼突起[17]。
载体和菌株:pCAMBIA1300-221双元植物表达载体、大肠杆菌(Escherichia coli)DH5α、根癌农杆菌(Agrobacterium tumefaciens)EHA105,由黑龙江八一农垦大学马铃薯研究所保存。
毒源马铃薯KX-PVX、PVYN和351-PSTVd,由黑龙江省农业科学院马铃薯研究所病毒实验室提供。
1.2 P25、HC-Pro、Virp1基因序列获得
TRIzol法分别提取感染PVX、PVY、PSTVd的马铃薯植株RNA,并反转录成cDNA。根据NCBI中公布的P25、HC-Pro、Virp1基因序列分别设计引物,分别为:P25-F(5′-TGTCACCGACGTGGGGTAG-3′),P25-R(5′-TGACCGGAGCGGTCAGTCT-3′);HC-Pro-F(5′-GCTGCCGACTCAGACATTAT-3′),HC-Pro-R(5′-TGCATTAGGAACACCACCAAG-3′);Virp1-F(5′-CGGAACTAAACTCGTGGTTC-3′),Virp1-R(5′-TGGAACCGCAGTTGGTTCCT-3′)。进行RT-PCR扩增,并将扩增产物进行回收测序。
1.3 靶向P25、HC-Pro、Virp1人工miRNA前体的构建
本试验前体骨架选择拟南芥 miR159a,以拟南芥DNA(CATB法提取)为模板,扩增拟南芥pre-miR159a,引物为:miR159a-F(5′-CGATAGATCTTGA
TCTGACGATGG-3′)和miR159a-R(5′-GAGAAGGTG
AAAGAAGATGTAGAG-3′)。
利用在线软件WMD(http://wmd2.weigelworldorg/)设计针对P25、HC-Pro、Virp1基因的amiRNA,序列分别为5′-ATAGTGACTACTTTGAACTCA-3′(P25);5′-TCGACTAGTATTCTAGGCAGT-3′(HC-Pro);5′-ATCTCTTACAATTAAGGCGAG-3′(Virp1)。
以拟南芥DNA为模板,通过PCR把拟南芥miR319a的21个核苷酸分别替换为P25、HC-Pro、Virp1基因的amiRNA,引物序列见表1。
表1 pre-amiR-P25、pre-amiR-HCPro、pre-amiR-Virp1引物
Tab.1 Sequence primers of pre-amiR-P25,pre-amiR-HCPro,pre-amiR-Virp1
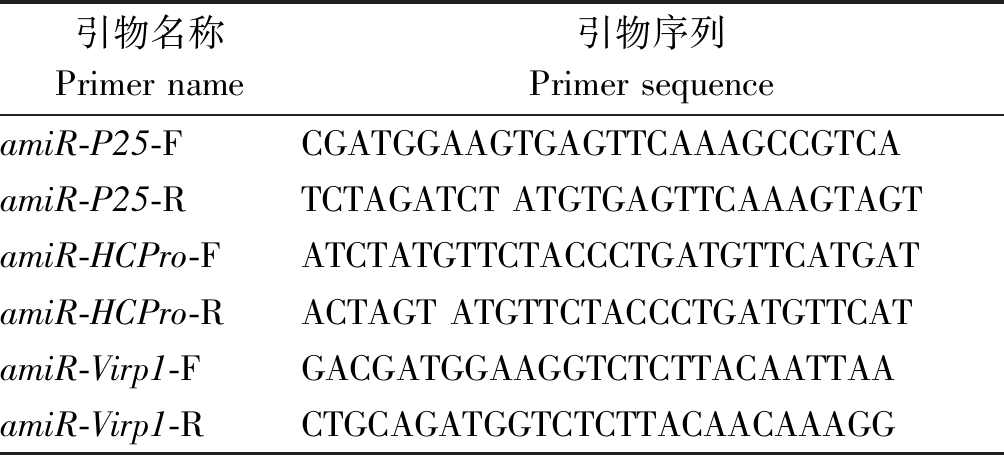
引物名称Primer name引物序列Primer sequenceamiR-P25-FCGATGGAAGTGAGTTCAAAGCCGTCAamiR-P25-RTCTAGATCT ATGTGAGTTCAAAGTAGTamiR-HCPro-FATCTATGTTCTACCCTGATGTTCATGATamiR-HCPro-RACTAGT ATGTTCTACCCTGATGTTCATamiR-Virp1-FGACGATGGAAGGTCTCTTACAATTAAamiR-Virp1-RCTGCAGATGGTCTCTTACAACAAAGG
1.4 pre-amiR-P25、pre-amiR-HCPro、pre-amiRNA-Virp1序列连接
为使pre-amiR-P25、pre-amiR-HCPro、pre-amiR-Virp1 3段序列连入pCAMBIA1300-211载体,分别在pre-amiRNA-P25中引入EcoR Ⅰ、Bgl Ⅱ酶切位点,pre-amiR-HCPro引入Spe Ⅰ、Bgl Ⅱ酶切位点,pre-amiRNA-Virp1引入Spe Ⅰ、Pst Ⅰ酶切位点,并进行PCR反应,结束后分别回收产物并保存。
采用over-lapping PCR将pre-amiR-P25、pre-amiR-HCPro和pre-amiR-Virp1 3段序列连接,在pre-amiRNA-P25中引入EcoR Ⅰ和Bgl Ⅱ酶切位点,pre-amiR-HCPro中引入Bgl Ⅱ和Spe Ⅰ酶切位点,pre-amiR-Virp1引入Spe Ⅰ和Pst Ⅰ酶切位点,连接过程见图1。将连接片段命名为pre-amiR-P25-HCPro-Virp1转化大肠杆菌DH5α,并连入pMD18-T载体,对重组质粒进行PCR鉴定。
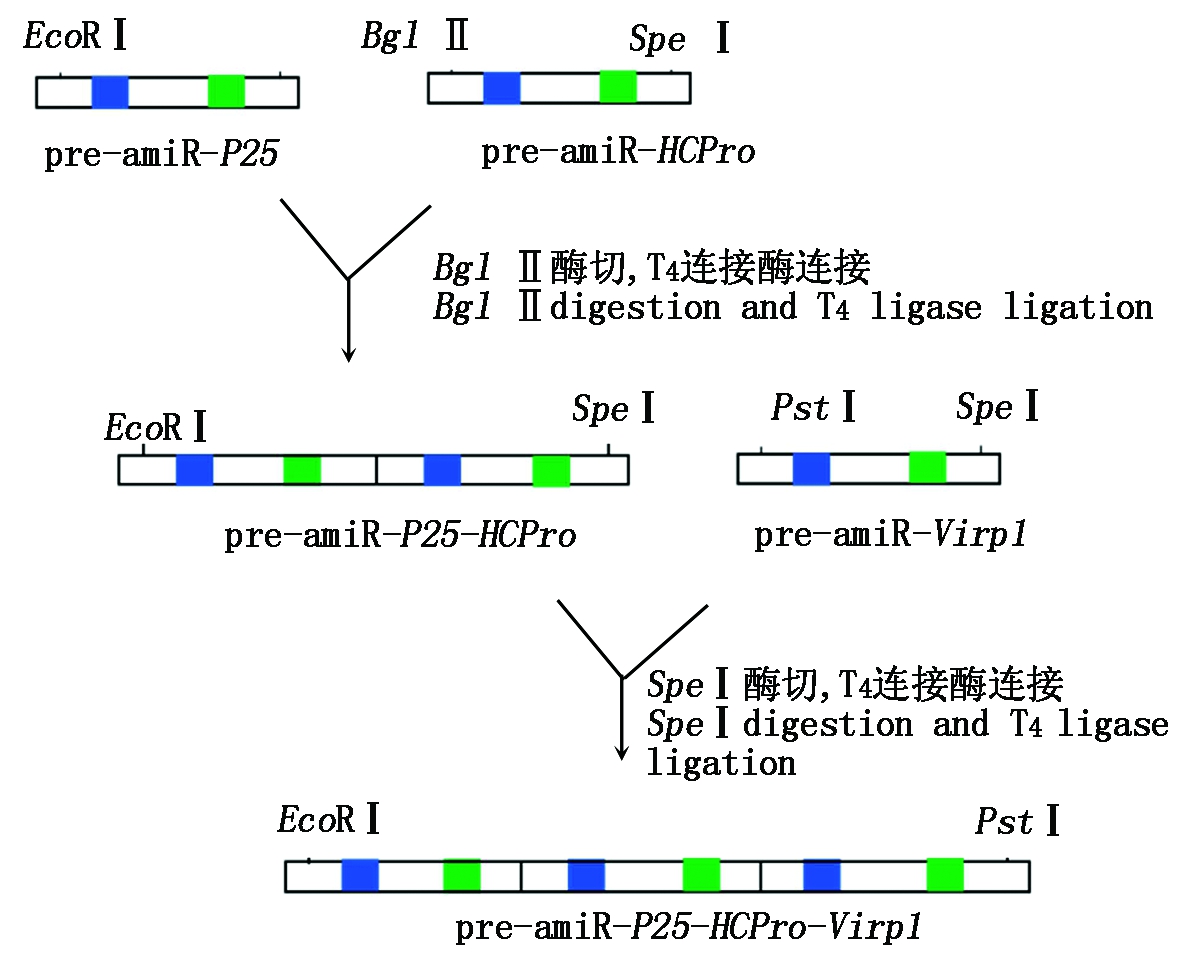
图1 pre-amiR-P25、pre-amiR-HCPro、pre-amiR-Virp1序列连接过程
Fig.1 Pre-amiR-P25,pre-amiR-HCPro andpre-amiR-Virp1 sequence connection process
1.5 pre-amiR-P25-HCPro-Virp1植物表达载体构建
将pCR回收产物与含pCAMBIA1300-221载体的质粒,分别用EcoR Ⅰ和Pst Ⅰ双酶切pMD18-T-pre-amiR-P25-HCPro-Virp1质粒和pCAMBIA1300-221载体,37 ℃酶切2~4 h。将质粒与载体酶切产物按3∶1的比例混合,用T4 连接酶16 ℃连接过夜;连接产物转化大肠杆菌DH5α,将重组质粒命名为p1300-221-amiR-P25-HCPro-Virp1,并对重组质粒进行PCR鉴定与酶切鉴定,阳性重组载体质粒电击法转化农杆菌EHA105感受态细胞。
1.6 根癌农杆菌介导的马铃薯脱毒微型薯片遗传转化
取度过休眠期的费乌瑞它脱毒微型薯,自来水冲洗30 min,酒精浸泡30 s,次氯酸钠消毒20 min,无菌纯净水冲洗3~4遍后,置于超净工作台中,去皮将薯肉切成1.0 cm×1.0 cm×0.2 cm的方块。侵染含p1300-221-amiR-P25-HCPro-Virp1载体农杆菌菌液,具体方法参照姜丽丽[18]进行。将侵染后的微型薯片接种至分化培养基中(MS+0.5 mg/L IAA(吲哚乙酸)+ 4 mg/L ZT(玉米素)+0.25 mg/LGA3(赤霉素)+300 mg/L Cef(头孢霉素)+50 mg/L Hyg(潮霉素)),培养条件为(24±2)℃,光照强度为2 000 lx、光照周期为光16 h/暗8 h;7 d 后转入抗性筛选培养基中(分化培养基中添加50 mg/L Hyg)培养,培养条件不变。
1.7 转amiR-P25-HCPro-Virp1植株PCR检测
提取转化amiR-P25-HCPro-Virp1费乌瑞它潮霉素筛选阳性植株DNA,以未转化植株作为阴性对照,上下游引物分别为amiR-P25-F和amiR-Virp301-R,进行 PCR扩增,反应条件为94 ℃预变性 5 min;94 ℃变性 30 s,58 ℃退火30 s,72 ℃延伸 30 s,30个循环;72 ℃延伸 10 min,以1%琼脂糖凝胶电泳检测。
1.8 转基因植株荧光定量PCR表达分析
为检测费乌瑞它转化植株中amiRNA的表达量,用荧光定量PCR (qRT-PCR)检测amiR-P25-HCPro-Virp1基因在转基因植株中的表达情况。以马铃薯的看家基因 StEF-1α 作为内参基因,内参基因引物序列为:StEF-1α-F(ATTGGAAACGGATATGCTCCA),StEF-1α-R(TCCTTACCTGAACGCCTGTCA),目的基因引物为amiR-P25-F和amiR-Virp301-R。
根据SYBR Premix Ex TaqTM的说明书要求,在荧光定量PCR仪(Bio-Rad,CFX-96)上进行,3次重复。PCR体系含2×SYBR Premix Ex Taq 10 μL,10 μmol/L引物各0.2 μL,cDNA模板2 μL,加RNase-free ddH2O至终体积20 μL。PCR条件为95 ℃预变性 3 min;95 ℃变性7 s,61 ℃退火10 s,72 ℃延伸15 s,45个循环;68 ℃延伸5 min。以1%琼脂糖凝胶电泳检测。数据由荧光定量PCR仪自动读取,采用2-ΔΔCt法对结果进行相对定量分析,计算amiRNA的表达水平。
1.9 转amiR-P25-HCPro-Virp1植株的抗病性鉴定
1.9.1 试管苗移栽 费乌瑞它阳性转化植株及未转化对照移栽至装有蛭石和珍珠岩的花盆中,并转移至网棚中培养,定期喷施Hoagland营养液壮苗,保持温室温度在25~30 ℃,每天光照12 h。
1.9.2 汁液摩擦法接种PVX、PVY、PVSTd病毒 取PVX、PVY、PVSTd毒源植株叶片各2 g置于灭菌研钵中加入20 mL的研磨缓冲液(1×PBS Buffer,pH值7.4)研磨成匀浆备用,转化植株长至6~8片叶时,在植株上部完全展开的叶片上均匀喷洒过直径为20 μm筛的金刚砂,用消毒毛刷蘸取少量混合毒源汁液均匀涂抹在费乌瑞它转化植株及未转化植株(CK)嫩叶上,接毒120 min后用无菌超纯水将叶片清洗干净。定期观察植株的症状,并对植株进行RT-PCR分子检测。
根据PVX、PVY和PSTVd的基因保守序列,设计引物,序列见表2。其中PVX目的片段为711 bp,PVY目的片段为447 bp,PSTVd目的片段为356 bp。
表2 PVX、PVY及PSTVd病毒检测引物
Tab.2 Primers of PVX,PVY and PSTVd virus detection
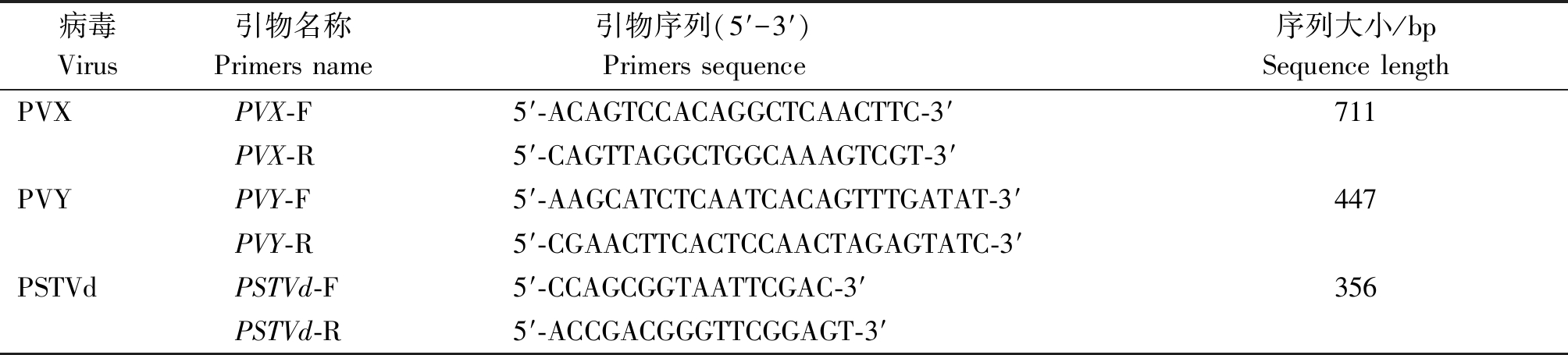
病毒Virus引物名称Primers name引物序列(5′-3′) Primers sequence 序列大小/bpSequence length PVXPVX-F5′-ACAGTCCACAGGCTCAACTTC-3′711PVX-R5′-CAGTTAGGCTGGCAAAGTCGT-3′PVYPVY-F5′-AAGCATCTCAATCACAGTTTGATAT-3′447PVY-R5′-CGAACTTCACTCCAACTAGAGTATC-3′PSTVdPSTVd-F5′-CCAGCGGTAATTCGAC-3′356PSTVd-R5′-ACCGACGGGTTCGGAGT-3′
2 结果与分析
2.1 amiR-P25、amiR-HCPro、amiR-Virp1前体的构建
以拟南芥DNA为模板,特异性引物(amiR-P25-F/amiR-P25-R,amiR-HCPro-F/amiR-HCPro-R,amiR-Virp1-F/amiR-Virp1-R)分别进行PCR扩增,将拟南芥pre-miR159a分别改造为pre-amiR-P25、pre-amiR-HCPro、pre-amiR-Virp1,均得到大小为239 bp的条带(图2),与所预测的片段大小相符。
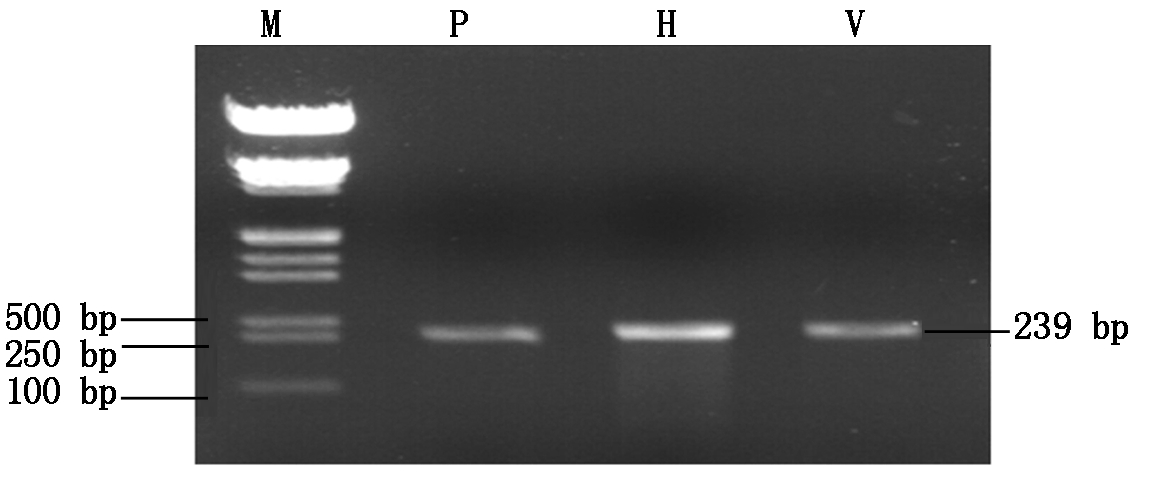
M.Marker(DL5000);P.pre-amiR-P25序列;
H.pre-amiR-HCPro序列;V.pre-amiR-Virp1序列。
M.Marker (DL5000);P.Sequence of pre-amiR-P25;H.Sequence of pre-amiR-HCPro;V.Sequence of pre-amiR-Virp1.
图2 pre-amiR-P25、pre-amiR-HCPro、preamiR-Virp1电泳检测
Fig.2 Result of agarose gel electrophoresis of pre-amiR-P25,pre-amiR-HCPro and pre-amiR-Virp1
2.2 p1300-221-amiR-P25-HCPro-Virp1植物表达载体的构建
连接后的pre-amiR-P25-HCPro-Virp1序列克隆到pMD18-T载体上,为了与目的载体连接,扩增序列时在引物中引入了EcoR Ⅰ和Pst Ⅰ位点。本试验采用的植物表达载体pCAMBIA1300-221双元载体,构建载体时用EcoR Ⅰ和Pst Ⅰ双酶切p1300-221质粒及pMD18-T-pre-amiR-P25-HCPro-Virp1,回收两片段并进行连接,经PCR及不同的酶切验证,结果表明载体构建正确(图3)。p1300-221-pre-amiR-P25-HCPro-Virp1载体构建图谱见图4。
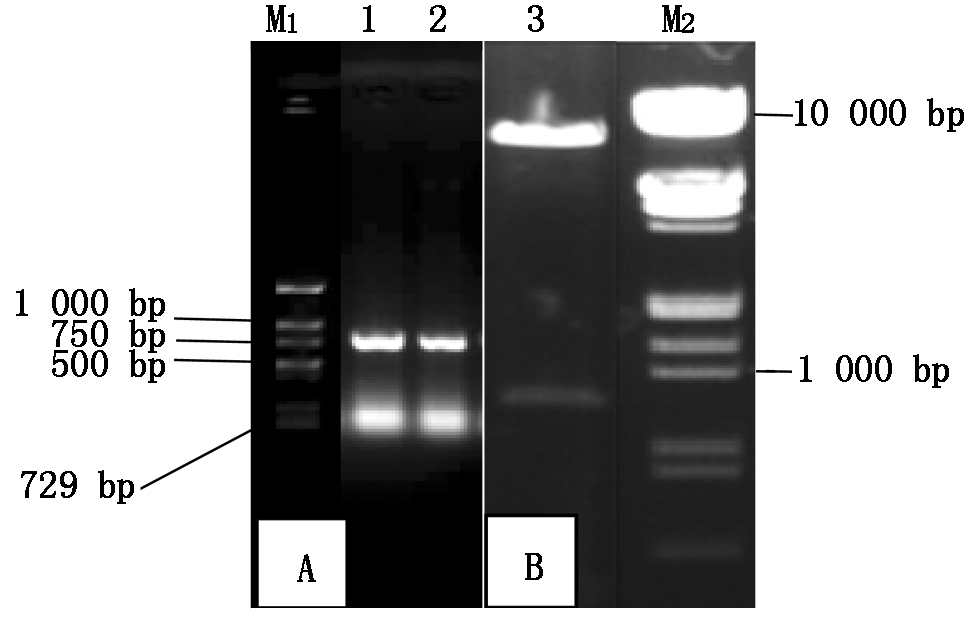
A.p1300-221-pre-amiR-P25-HCPro-Virp1表达载体PCR验证:M1.Marker(DL1500);1~2.表达载体PCR检测结果。B:M2.Marker(DL15000);3.EcoRⅠ和Pst Ⅰ双酶切验证。
A.PCR results of p1300-221-pre-amiR-P25-HCPro-Virp1:M1.Marker(DL1500);1-2.PCR results of expression vector.B:M2.Marker(DL15000);3.Identified by enzyme digestion of EcoRⅠ and Pst Ⅰ.
图3 p1300-221-pre-amiR-P25-HCPro-Virp1表达载体验证
Fig.3 Verification of p1300-221-pre-amiR-P25-HCPro-Virp1 expression vector
2.3 转amiR-P25-HCPro-Virp1植株获得
农杆菌侵染后的费乌瑞它微型薯片在分化培养基中培养14 d后,薯块边缘逐渐泛绿,21 d后,薯片颜色加深,转为深绿,当培养至28 d左右时,在薯块表面形成多个生长点,继续培养至生长点发展成再生芽,再生芽长至1.5~2.0 cm时,剪下来接入添加50 mg/L Hyg的MS培养基中(图5)。
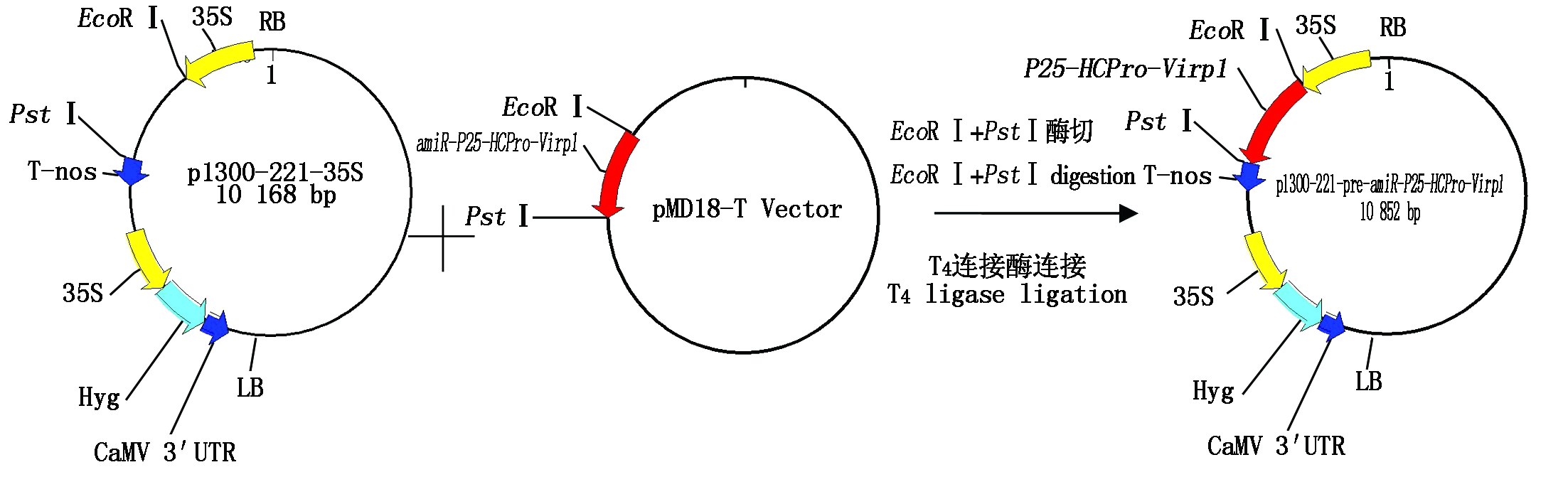
图4 p1300-221-pre-amiR-P25-HCPro-Virp1表达载体的构建过程
Fig.4 Construction process of p1300-221-pre-amiR-P25-HCPro-Virp1 expression vector
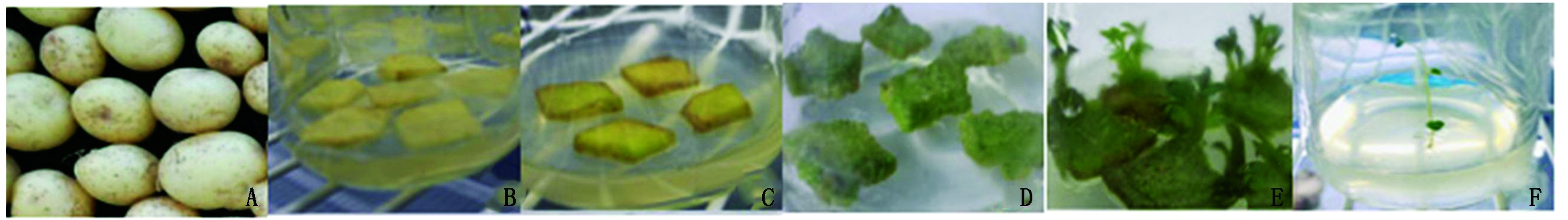
A.费乌瑞它微型薯;B.微型薯片侵染;C.侵染14 d的微型薯片;D.接种21 d 微型薯片;
E.接种28 d 微型薯片;F.再生植株移至MS培养基中培养。
A.Minituber of Favortia;B.Infection of minituber slice;C. Infection of minituber slice for 14 days;D.Infection of minituber slice for 21 days;E. Infection of minituber slice for 28 days;F. The regenerated plants were transferred to MS medium for culture.
图5 费乌瑞它转化植株获得
Fig.5 Obtaining and transplanting transgenic plant of Favortia
2.4 T0植株PCR检测
费乌瑞它微型薯片经含amiR-P25-HCPro-Virp1质粒农杆菌侵染、再生、压力筛选,抗性植株分化及植株壮苗共获得转化株系15株,用amiR-P25-HCPro-Virp1特异性引物对转化株系PCR检测。检测结果如图6所示,有10个转化株系扩增出与质粒正对照同样大小约729 bp的片段,而未转化植株没有扩增片段,可以初步说明amiR-P25-HCPro-Virp1基因转化至马铃薯中。
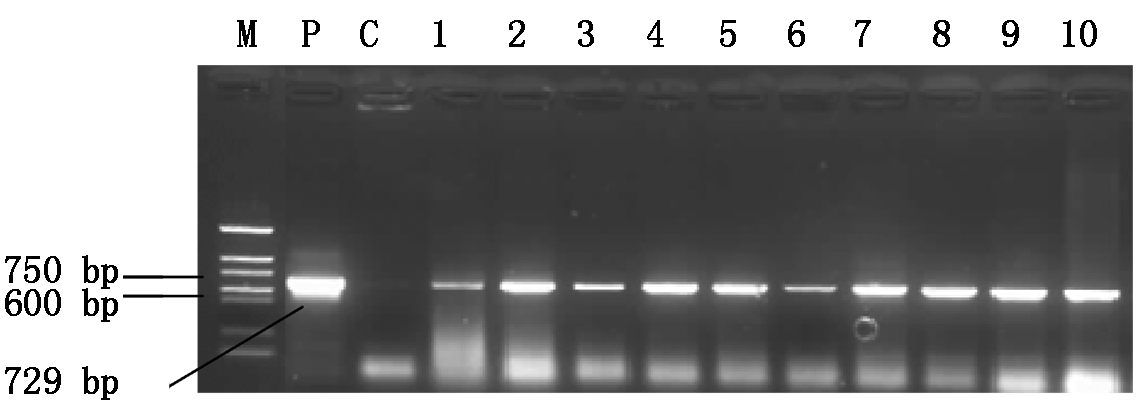
M.Marker(DL2000);P.质粒对照(+);
C.未转化植株对照(-);1-10.转化植株。
M.DNA Marker (DL2000); P. Plasmid CK;
C.Negative control of untransformed CK;1-10.Transgenic plants.
图6 转amiR-P25-HCPro-Virp1植株PCR鉴定
Fig.6 PCR analysis of the transgenic plants of amiR-P25-HCPro-Virp1
2.5 T0转化植株的qRT-PCR检测
为检测amiR-P25-HCPro-Virp1基因在马铃薯植株中的表达水平,对PCR阳性转化株系进行qRT-PCR检测。各转化株系相对表达量如图7所示,amiR-P25-HCPro-Virp1基因在10个转化植株中均有所表达,从相对表达量来看,各转化株系间相对表达量差异显著,9号转化株系相对表达量最高为21.37,1号转化株系最低为7.68。
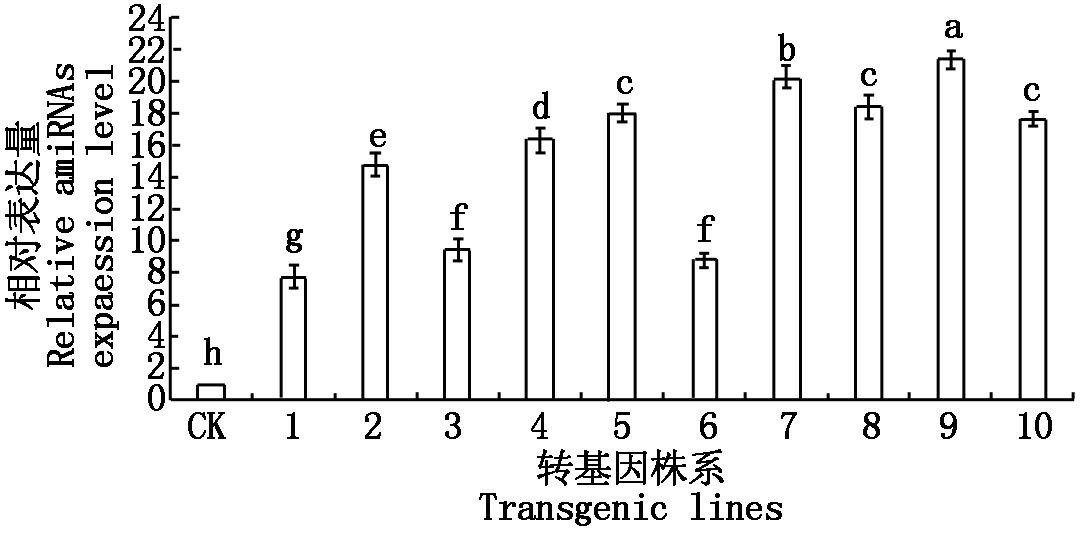
数据为3次重复平均值,不同小写字母表示差异显著(P<0.05)。数据统计分析采用LSD法。
The data were the average of three replicates,and the difference was significant in different lowercase letters ( P<0.05 ). The LSD method was used for statistical analysis of the data.
图7 不同转基因株系中amiR-P25-HCPro-Virp1基因表达量
Fig.7 Transcription level of amiR-P25-HCPro-Virp1 in different transgenic lines
2.6 T0转化植株病毒抗性评价
PVX、PVY和PVSTd病毒等比例混合液摩擦接种转化植株感病情况如图8。接种病毒混合液后费乌瑞它未转化植株矮小,节间缩短,叶缘上卷,叶片斑驳;随着植株的生长感染植株会出现花期滞后,少量块茎表皮裂口等情况。转amiR-P25-HCPro-Virp1植株则生长正常,叶片嫩绿、平展,块茎表皮光滑。
接种病毒混合液20 d后取叶分别以PVX、PVY、PVSTd检测引物进行RT-PCR检测结果如图9,在未转化对照植株中分别检测到与PVX病毒基因长度一致的711 bp片段(图9-A),与PVY病毒基因长度一致的450 bp片段(图9-B),与PVSTd病毒基因长度一致的356 bp片段(图9-C),而接种病毒混合液的转化植株未出现任何扩增条带。这表明,转amiR-P25-HCPro-Virp1基因植株对PVX、PVY、PVSTd这3种病毒具有抗性。
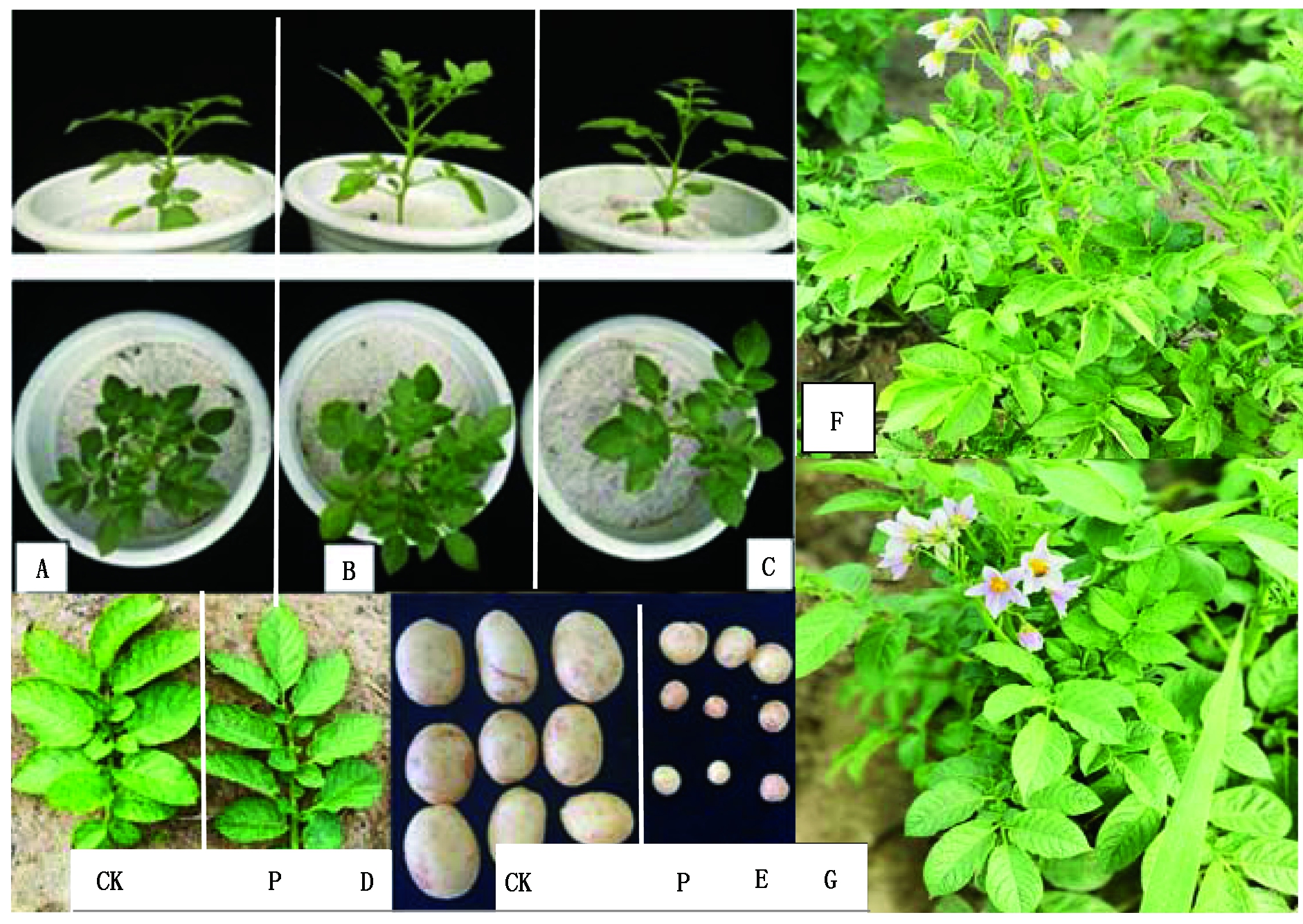
A.未转化植株(CK);B-C.费乌瑞它转化植株;D.转化植株与对照植株叶片比较;
E.转化植株与对照植株块茎比较;F.对照植株田间生长情况;G.转化植株田间生长情况。
A.Non transformed plants (CK);B-C.Transgenic plants of Favorita;D.Comparison of leaves between transgenic plants and CK;E.Comparison of tubers between transgenic plants and CK;F. Field growth of non-transformed plants;G.Field growth of non-transformed plants.
图8 T0转化植株病毒抗性检测
Fig.8 Test of virus resistance in transformed T0 plants
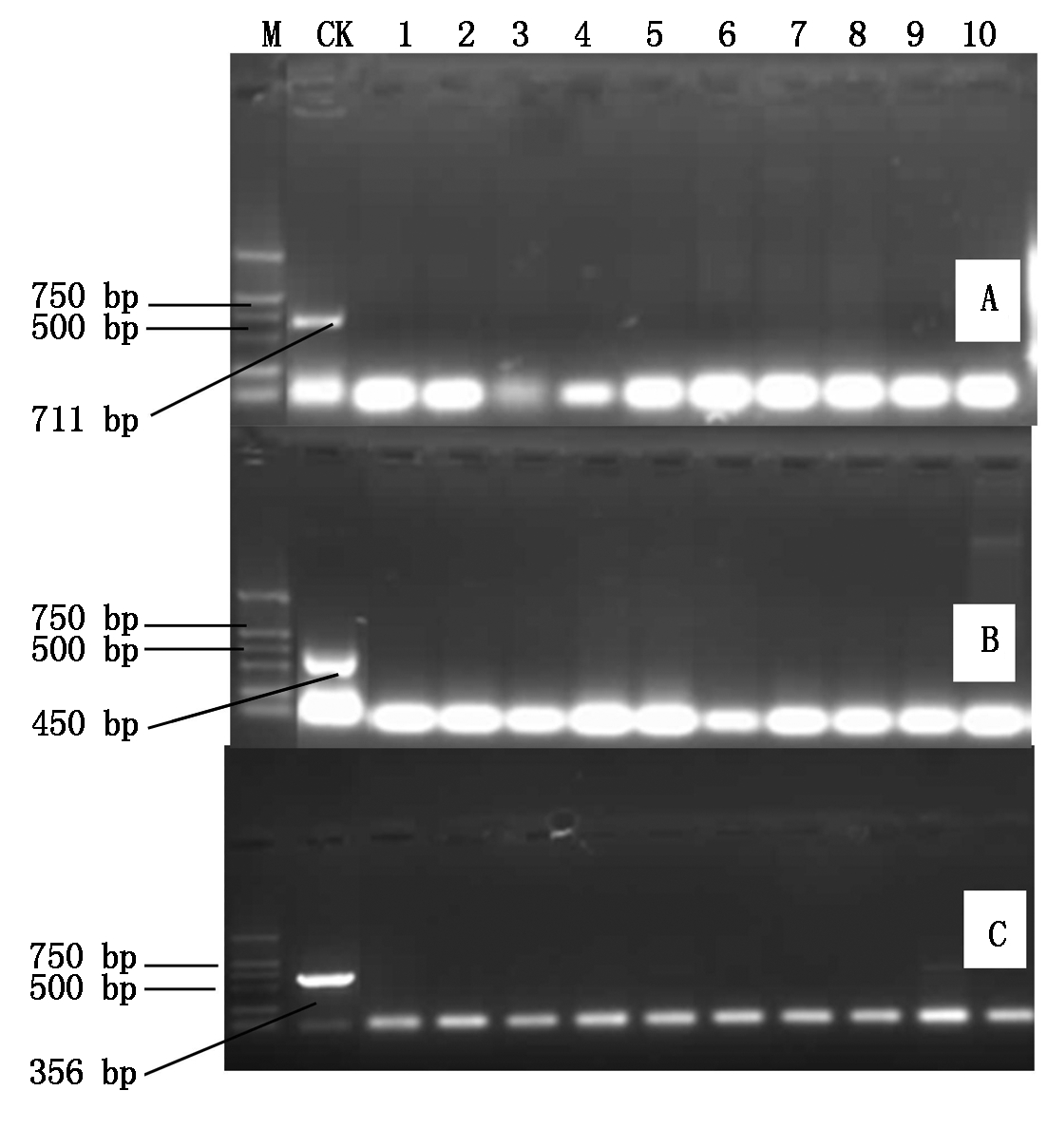
A.转化植株PVX病毒检测结果;B.转化植株PVY病毒检测结果;C.转化植株PVSTd病毒检测结果;M.Marker;CK.接种病毒混合液的未转化植株;1-10.接种病毒混合液的费乌瑞它转化植株。
A.PVX virus detection results of transgenic plants;B.PVY virus detection results of transgenic plants;C.PVSTd virus detection results of transgenic plants;M.Marker;CK.Non transgenic plants by inoculating with virus mixture;1-10.Transgenic plants of Favorita by inoculating with virus mixture.
图9 T0转化植株的RT-PCR病毒检测结果
Fig.9 Verification of T0 transgenic plants by RT-PCR for virus infection
3 结论与讨论
合适的植物内源miRNA前体骨架是获得amiRNA前体的基础[19]。已有研究表明,miR156、miR159、miR164、miR165、miR167、miR169、miR171、miR172、miR319、miR528等多个植物内源miRNA被用于构建人工miRNA骨架[20-24]。 尽管在amiRNA构建中最好是用自身固有的miRNA前体作为骨架,然而目前已被证实功能的前体骨架中并没有马铃薯内源miRNA前体,而拟南芥中已有多个miRNA前体骨架被证明具有加工功能且已经应用到多种外源作物中,艾涛波[25]通过比较拟南芥miR159a、miR167b、miR171a 3种前体骨架沉默效率结果表明,与pre-miR167b和pre-miR171a相比,miR159a的茎环结构更稳定,更容易被DCL1 所识别,提高了miR159a沉默靶基因的效率,且以miR159a为前体构建的靶向体用于抗PVX和PVY的amiRNA已有研究,本研究以拟南芥miR159a为前体骨架分别改造并构建了amiR-P25、amiR-HCPro和amiR-Virp1,为进一步构建兼抗PVX、PVY和PSTVd表达载体奠定基础。
人工miRNA序列与目的基因靶序列之间的互补性对于转化植株的抗病能力至关重要[26-27]。本研究设计P25、HC-Pro、Virp1基因人工miRNA序列时利用在线软件WMD进行设计同时也参考了这3个基因测序中出现频率较高的位点,最大程度保证了沉默效率。自amiRNA技术应用于植物基因工程以来,己有多项研究报道了向植物体中转入amiRNA不仅可以改变植物的表型特征,还能使植物获得抗病毒特性以及其他优良特性,如烟草[28]、黄瓜[29]、草莓[30]、番茄[13],马铃薯[31]等。如果将2种或2种以上针对不同病毒基因的amiRNA转入同一植物中,可同时沉默多个基因,进而使植物获得2种或2种以上病毒的抗性[32]。Ai等[33]构建了靶向PVX、PVY的amiR-P25-HC-Pro表达载体转化烟草,使转化植株获得同时抗 PVX 和 PVY 的双重抗性。Kung等[34]将西瓜银斑驳病毒(Watermelon silver mottle virus WSMoV)中的5个靶向区段A、B1、B2、C、D和 E中的AB1E和B2DC构建了amiRNA表达载体并转化烟草,结果表明转化植株对WSMoV具有完全抗性。
已有研究表明,PVX和PVY复合侵染会产生协同作用而使植株的病情加重[35],PVY和PSTVd混合感染会导致马铃薯产量下降加剧[17]。本研究中,费乌瑞它未转化植株感染病毒混合液后同时检测到了PVX、PVY和PSTVd,这表明病毒混合液中3种病毒(类病毒)均具感染性,本研究未对非转化植株进行PVX、PVY和PSTVd的定量分析,因此,无法判断三者之间感染力的差别。在今后工作中,将进一步完善此方面的研究。此外,本研究仅对T0转化株系进行了抗性研究,且转化株系表现出兼抗PVX、PVY和PSTVd的特性,然而Petchthai等[36]在amiRNA获得兼抗烟草研究建兰花叶病毒 (CymMV)和齿兰环斑病毒 (ORSV) 中发现,F4转基因烟草对CymMV表现100%抗性,而对ORSV仅有16%的抗性。分析原因,可能与前体骨架选择及病毒中存在沉默抑制子有关。尽管本研究优选拟南芥miR159a为前体骨架,且设计amiRNA时针对3种病毒(类病毒)的沉默抑制子,仍会继续评价高世代转化植株兼抗病毒特性和稳定性。
本研究针对马铃薯PVX、PVY、PVSTd蛋白的P25、HC-Pro、Virp1基因,以拟南芥pre-miR159a为骨架分别构建靶向这3个基因的amiRNA,并构建植物表达载体p1300-221-pre-amiR-P25-HCPro-Virp1,农杆菌介导法转化费乌瑞它脱毒微型薯片,经抗性筛选、PCR检测共获得10株阳性转化植株,荧光定量PCR结果表明,外源基因在阳性转化植株中均有表达。对T0阳性转化植株摩擦接种病毒混合液接结果表明,转化植株对PVX、PVY和PVSTd混合感染下具有兼抗的特性。
[1] 张玉胜.中国马铃薯产品国际竞争力及出口潜力研究[D].北京:中国农业科学院,2020.doi:10.27630/d.cnki.gznky.2020.000468.
Zhang Y S. International competitiveness and export potential of potato products in China[D].Bejing:Chinese Academy of Agricultural Sciences,2020.
[2] 陈虞超,聂峰杰,张丽,巩檑,甘晓燕,石磊,宋玉霞.马铃薯X病毒研究进展[J]. 长江蔬菜,2016(18):39-44.doi:10.3865/j.issn.1001-3547.2016.18.015.
Chen Y C,Nie F J,Zhang L,Gong L,Gan X Y,Shi L,Song Y X. Research progress of Potato virus X[J]. Journal of Changjiang Vegetables,2016(18):39-44.
[3] 张华鹏,张剑峰,刘俊莹,王聪聪. 马铃薯上PVY、PVS和PLRV的三重RT-PCR检测[J]. 华北农学报,2011,26(5):40-45. doi:10.7668/hbnxb.2011.05.009.
Zhang H P,Zhang J F,Liu J Y,Wang C C. Triplex-RT-PCR detection of PVY,PVS and PLRV in potato[J].Acta Agriculturae Boreali-Sinica,2011,26(5):40-45.
[4] 贺振,陈春峰,张志想,李世访.马铃薯Y病毒科分子进化研究进展[J].植物保护,2017,43(3):13-22. doi:10.3969/j.issn.0529-1542.2017.03.003.
He Z,Chen C F,Zhang Z X,Li S F.Advances in molecular evolution of viruses in the family Potyviridae[J].Plant Protection,2017,43(3):13-22.
[5] Flores R,Hernández C,de Alba A E M,Daròs J A,Serio F D. Viroids and viroid-host interactions[J].Annual Review of Phytopathology,2005,43(1):117-139. doi:10.1146/annurev.phyto.43.040204.140243.
[6] 范国权,白艳菊,高艳玲,张威,张抒,申宇,刘凯,喻江.中国马铃薯主要病毒病发生情况调查与分析[J].东北农业大学学报,2013,44(7):74-79. doi:10.3969/j.issn. 1005-9369.2013.07.014.
Fan G Q,Bai Y J,Gao Y L,Zhang W,Zhang S,Shen Y Liu K,Yu J.Investigation and analysis on potato viral disease in China[J].Journal of Northeast Agricultural University,2013,44(7):74-79.
[7] 邱彩玲,吕典秋,董学志,魏琪,刘尚武,王绍鹏,宿飞飞,李勇,白艳菊. 我国在防治马铃薯类病毒病中存在的问题及防治对策[J].东北农业大学学报,2011,42(10):140-144. doi:10.3969/j.issn.1005-9369.2011.10.029.
Qiu C L,Lü D Q,Dong X Z,Wei Q,Liu S W,Wang S P,Su F F,Li Y,Bai Y J. Problems and countermeasures on potato spindle tuber viroid (PSTVd) control in China[J].Journal of Northeast Agricultural University,2011,42(10):140-144.
[8] Zhao J P,Rios C G,Song J Q. Potato virus X-based microRNA silencing (VbMS) in potato[J]. Journal of Visualized Experiments,2020(159):e61067. doi:10.3791/61067.
[9] 许宗宏,郝青南,陈李淼,孙佃臣,田星星,单志慧.基于大豆花叶病毒衣壳蛋白基因的RNA干扰植物表达载体的构建[J].华北农学报,2010,25(S2):1-4. doi:10.7668/hbnxb.2010.S2.001.
Xu Z H,Hao Q N,Chen L M,Sun D C,Tian X X,Shan Z Z. Plant expression construct based on RNAi for Soybean mosaic virus[J]. Acta Agriculturae Boreali-Sinica,2010,25(S2):1-4.
[10] Zhao L Q,Li H L,Li R,Li W,Hua J P,Guo Y D. Cloning of cotton delta-12 oleate desaturase gene FAD2-1 and construction of its ihpRNA and amiRNA interference vectors[J].Agricultural Science & Technology,2012,13(11):2281-2283,2286. doi: 10.1617 5/j.cnki.1009-4229.2012.11.028.
[11] 周香艳,杨江伟,唐勋,文义凯,张宁,司怀军. amiRNA技术沉默C-3氧化酶编码基因StCPD对马铃薯抗旱性的影响[J].作物学报,2018,44(4):512-521.doi: 10.3724/SP.J.1006.2018.00512.
Zhou X Y,Yang J W,Tang X,Wen Y K,Zhang N,Si H J. Effect of silencing C-3 oxidase encoded gene StCPD on potato drought resistance by amiRNA technology[J].Acta Agronomica Sinica,2018,44(4):512-521.
[12] Carbonell A,Daròs J A. Artificial microRNAs and synthetic trans-acting small interfering RNAs interfere with viroid infection[J].Molecular Plant Pathology,2017,18(5):746-753. doi:10.1111/mpp.12529.
[13] Sharma N,Prasad M. Silencing AC1 of Tomato leaf curl virus using artificial microRNA confers resistance to leaf curl disease in transgenic tomato[J].Plant Cell Reports,2020,39(11):1565-1579. doi:10.1007/s00299-020-02584-2.
[14] Meyers B C,Axtell M J. MicroRNAs in plants:key findings from the early years[J]. The Plant Cell,2019,31(6):1206-1207. doi:10.1105/tpc.19.00310.
[15] Zubair M,Khan M Z,Rauf I,Raza A,Shah A H,Hassan I,Amin I,Mansoor S. Artificial micro-RNA (amiRNA)-mediated resistance against whitefly(Bemisia tabaci) targeting three genes [J].Crop Protection,2020,137:105308. doi:10.1016/j.cropro.2020.105308.
[16] 范国权,高艳玲,张威,张抒,申宇,邱彩玲,白艳菊,刘凯,喻江.马铃薯主要病毒侵染不同品种症状及对产量的影响[J]. 中国马铃薯,2019,33(1):34-42. doi:10.3969/j.issn.1672-3635.2019.01.006.
Fan G Q,Gao Y L,Zhang W,Zhang S,Shen Y,Qiu C L,Bai Y J,Liu K,Yu J. Symptoms and yields of different Potato varieties infected with main potato viruses[J].Chinese Potato Journal,2019,33(1):34-42.
[17] 邱彩玲,吕文河,吕典秋,白艳菊,魏琪,刘尚武,董学志,耿宏伟,万书明,魏峭嵘.4个马铃薯品种感染马铃薯纺锤块茎类病毒(PSTVd)的症状[J].植物保护,2014,40(6):159-163.doi:10.3969/j.issn.0529-1542.2014.06.030.
Qiu C L,Lü W H,Lü D Q,Bai Y J,Wei Q,Liu S W,Dong X Z,Geng H W,Wan S M,Wei Q R. Symptoms of four potato varieties infected with Potato spindle tuber viroid (PSTVd)[J].Plant Protection,2014,40(6):159-163.
[18] 姜丽丽.农杆菌介导的BcBCP1基因转化马铃薯抗旱性研究[D].哈尔滨:东北农业大学,2008:21-22. doi:10.7666/d.y1403917.
Jiang L L. Agrobacterium-mediated transformation of potato with drought resistance gene BcBCP1[D]. Harbin:Northeast Agricultural University,2008:21-22.
[19] Kis A,Tholt G,Ivanics M,Várallyay É,Jenes B,Havelda Z. Polycistronic artificial miRNA-mediated resistance to Wheat dwarf virus in barley is highly efficient at low temperature[J].Molecular Plant Pathology,2016,17(3):427-437.doi:10.1111/mpp. 12291.
[20] Sánchez-Gutiérrez A,Ovando-Medina I,Adriano-Anaya L,Vázquez-Ovando A,Salvador-Figueroa M.Dynamics of miR156 and miR172 involved in the flowering of Jatropha curcas L.[J]. Acta Botanica Brasilica,2018,32(1):99-106.doi:10.1590/0102-33062017abb0179.
[21] Castrob  ,Quirozc D,Sáncheza E,de los
,Quirozc D,Sáncheza E,de los  ngeles Micconoa M,Aguirrea C,Ramírezd A,Montesa C,Prieto H.Synthesis of an artificial Vitis vinifera miRNA 319e using overlapping long primers and its application for gene silencing[J].Journal of Biotechnology,2016,233:200-210.doi:10.1016/j.jbiotec.2016.06.028.
ngeles Micconoa M,Aguirrea C,Ramírezd A,Montesa C,Prieto H.Synthesis of an artificial Vitis vinifera miRNA 319e using overlapping long primers and its application for gene silencing[J].Journal of Biotechnology,2016,233:200-210.doi:10.1016/j.jbiotec.2016.06.028.
[22] Wang X M,Yang Y,Yu C L,Zhou J,Cheng Y,Yan C Q,Chen J P. A highly efficient method for construction of rice artificial microRNA vectors[J].Molecular Biotechnology,2010,46(3):211-218. doi:10.1007/s12033-010-9291-4.
[23] Liu C,Zhang L,Sun J,Luo Y Z,Wang M B,Fan Y L,Wang L.A simple artificial microRNA vector based on ath-miR169d precursor from Arabidopsis[J].Molecular Biology Reports,2010,37(2):903-909.doi:10.1007/s11033-009-9713-1.
[24] Li H,Deng Y,Wu T L,Subramanian S,Yu O. Misexpression of miR482,miR1512,and miR1515 increases soybean nodulation[J].Plant Physiology,2010,153(4):1759-1770. doi:10.1104/pp.110.156950.
[25] 艾涛波. amiRNA介导抗性转基因烟草植株的抗病性分析[D].泰安:山东农业大学,2010:82-83. doi:10.7666/d.y178 6172.
Ai T B. Analysis of the resistance mediated by amiRNA silencing in transgenic tobacco plants[D].Taian:Shangdong Agricultural University,2010:82-83.
[26] Brodersen P,Sakvarelidze-Achard L,Bruun-Rasmussen M,Dunoyer P,Yamamoto Y Y,Sieburth L,Voinnet O.Widespread translational inhibition by plant miRNAs and siRNAs[J].Science,2008,320(5880):1185-1190. doi:10.1126/science.1159151.
[27] Meister G,Landthaler M,Patkaniowska A,Dorsett Y,Teng G,Tuschl T.Human Argonaute2 mediates RNA cleavage targeted by miRNAs and siRNAs[J].Molecular Cell,2004,15(2):185-197.doi:10.1016/j.molcel.2004.07.007.
[28] Zhao J P,Liu Q T,Hu P,Jia Q,Liu N,Yin K Q,Cheng Y,Yan F,Chen J P,Liu Y L.An efficient Potato virus X-based microRNA silencing in Nicotiana benthamiana[J]. Scientific Reports,2016,6:20573. doi:10.1038/srep20573.
[29] 梁超琼.响应黄瓜绿斑驳花叶病毒侵染的黄瓜miRNA功能分析及人工miRNA介导的病毒抗性评价[D]. 北京:中国农业大学,2018.
Liang C Q. Functional analysis of Cucumber green mottle mosaic virus-responsive miRNA in cucumber and evaluation of artificial miRNA-mediated virus resistance[D].Bejing:China Agriculturai University,2018.
[30] Li H,Dong X X,Mao W J,Guan Y H,Zhang Z H. An effective artificial microRNA vector based on Fv-miR166 precursor from strawberry [J].Scientia Horticulturae,2019,256:108643.doi:10.1016/j.scienta.2019.108643.
[31] Li S G,Zhang N,Zhu X,Ma R,Yang J W,Tang X,Si H J. Enhanced drought tolerance with artificial microRNA-mediated StProDH1 gene silencing in potato [J].Crop Science,2020,60(3):1462-1471. doi:10.1002/csc2.20064.
[32] Zhang N N,Zhang D D,Chen S L,Gong B Q,Guo Y J,Xu L H,Zhang X N,Li J F.Engineering artificial MicroRNAs for multiplex gene silencing and simplified transgenic screen[J].Plant Physiology,2018,178(3):989-1001.doi:10.1104/pp.18.00828.
[33] Ai T,Zhang L,Gao Z,Zhu C X,Guo X. Highly efficient virus resistance mediated by artificial microRNAs that target the suppressor of PVX and PVY in plants[J].Plant Biology,2011,13(2):304-316.doi:10.1111/j.1438-8677.2010.00374.x.
[34] Kung Y J,Lin S S,Huang Y L,Chen T C,Harish S S,Chua N H,Yeh S D.Multiple artificial microRNAs targeting conserved motifs of the replicase gene confer robust transgenic resistance to negative-sense single-stranded RNA plant virus[J].Molecular Plant Pathology,2012,13(3):303-317.doi:10.1111/j.1364-3703.2011.00747.x.
[35] Hameed A,Tahir M N,Asad S,Bilal R, Van Eck J,Jander G,Mansoor S. RNAi-mediated simultaneous resistance against three RNA viruses in potato[J].Molecular Biotechnology,2017,59(2/3):73-83.doi:10.1007/s12033-017-9995-9.
[36] Petchthai U,Yee C S L,Wong S M. Resistance to CymMV and ORSV in artificial microRNA transgenic Nicotiana benthamiana plants[J].Scientific Reports,2018,8(1):9958.doi:10.1038/s41598-018-28388-9.