长江中下游地区是我国重要的水稻主产区,近年来,由于气候变暖导致的夏季极端高温天气时有发生,最高温度超过35 ℃的天气持续10~15 d。高温胁迫已对当地的水稻生产造成严重影响,并已成为制约当地水稻种植业发展的主要限制因素之一。高温胁迫会对不同生育时期的水稻生长造成影响,在种子萌发期遭遇超过35 ℃的高温胁迫会导致种子发芽迟缓、种子丧失活力、发芽率下降[1]。水稻在幼苗阶段遭受高温胁迫不仅会出现叶片卷曲,含水量降低,叶片颜色变浅、变白、变短和畸形等症状,还会致使植株生长发育缓慢、抑制根系生长[2-3],光合作用受阻,净光合速率和气孔导度降低,光合作用原初反应受到抑制[4-5]。水稻孕穗期遭遇高温胁迫会导致颖花退化、花粉败育、花粉活力下降,进而导致秕粒增加[6-7];在抽穗期和灌浆期遭受高温胁迫会阻碍水稻的开花授粉,穗中秕粒增多、结实率下降,造成千粒质量降低,进而导致水稻减产[8-11]。研究表明,水稻幼苗在碱胁迫环境下,根系活性氧大量积累,破坏了抗氧化防御系统,进而损伤根系细胞,导致幼苗萎蔫和死亡[12]。高温胁迫与碱胁迫类似,其抑制水稻生长发育的一个重要因素就是引起ROS过量积累,破坏ROS产生和清除的平衡体系,导致膜质过氧化作用加剧,进而损坏细胞结构[3,13-14]。高温胁迫抑制水稻种子萌发,但其导致发芽率下降的主要原因尚不明确,本研究拟以ROS积累为线索探究高温胁迫抑制水稻种子萌发的机制。
脱落酸(ABA)是植物体内的重要激素,常作为胁迫激素参与植物对多种逆境胁迫的响应,并发挥重要作用[15]。产生诱抗效应是ABA提高植物抗逆性的一个重要机制[16]。ABA对水稻耐盐碱胁迫产生诱抗效应,能够提高水稻对碱胁迫的抗性和苏打盐碱水田中的产量[17-18]。ABA在提高植物耐高温胁迫中同样具有促进作用,高温处理可降低植物体内IAA、GA、自由脯氨酸及可溶性蛋白质的含量,增加脱落酸含量[19]。抽穗期喷施S-诱抗素或ABA溶液能够提高水稻的结实率和千粒质量,稻米的碾磨品质和蒸煮品质也得到改良[20]。研究表明,外源ABA能够通过增加蔗糖的转运和加速蔗糖代谢来保持碳平衡和能量平衡,进而阻止花粉败育[21]。以上研究结果为ABA提高水稻耐高温特性的效果提供了坚实的科学依据,但目前对于其内在的生理机制及分子机理解析尚不明晰,尤其在ROS相关通路中的报道并不多见。研究表明,ABA预处理能够提高碱胁迫下水稻幼苗的下游抗氧化防御能力,抑制ROS过量积累;且ABA缓解了外源二氯百草枯(Paraquat)对水稻幼苗引发的氧化胁迫,这是ABA诱导水稻耐碱胁迫的主要途径之一[22]。基于以上研究结果,本研究以高温为主要非生物胁迫因子,探究ABA对水稻耐高温胁迫的诱抗效应,以ABA浸种的方式在水稻种子萌发期进行研究,并以ROS积累为出发点初步解析高温环境下ABA对水稻种子萌发的影响机理。
以江西省主推常规水稻品种黄华占和日本晴为试验材料。
1.2.1 ABA溶液的配制 脱落酸(ABA:Sigma,Inc.,St,Louis,MO,USA)试剂先溶于少量的无水乙醇中,然后用去离子水定容至一定的浓度。本试验用外源ABA浸种的方式探究ABA对水稻种子萌发的诱抗效应,参照前人研究结果,选用10 μmol/L作为试验用ABA浓度[17-18,22]。
1.2.2 试验设计 萌发试验在培养皿中进行,设置2个处理,即非ABA处理组(-ABA)和ABA处理组(+ABA),每个处理5次重复。每个重复选取饱满一致的50粒种子,ABA处理组用10 μmol/L的ABA溶液于黑暗条件下浸种24 h,非ABA处理组用去离子水于黑暗条件下浸种24 h。
经ABA或去离子水浸种后的水稻种子用蒸馏水清洗干净,选取饱满且大小一致的种子,随机播撒在铺有2层滤纸的培养皿(直径为9 cm)中,分别添加10 mL蒸馏水进行萌发。萌发试验在宜春学院生命科学与资源环境植物科学实验室的光照培养箱(型号:SPT-P150C)中进行,设置对照(CK)和高温(HS)2种环境,对照组(CK)置于30 ℃、16 h光/8 h暗的条件下进行,高温处理组(HS)置于40 ℃、16 h光/8 h暗的条件下进行。试验设置4个处理:对照(CK-ABA)、ABA浸种(CK+ABA)、高温处理(HS-ABA)和ABA浸种后施加高温处理(HS+ABA),期间不断补充水分。种子萌发以芽长等于种子长度的一半和根长等于种子长度为准,每隔24 h记录一次,于萌发的第2 天开始调查,萌发的第7 天统计最终发芽率,同时每个培养皿选择10株幼苗测量芽长和主根长,并测定幼芽的相对电导率。各处理取0.1 g新鲜的水稻幼芽,剪成0.3 cm的小段迅速放入RNase灭活的2 mL离心管中,液氮中速冻,并放入-80 ℃冰箱保存备用,分别用于丙二醛含量、ROS含量![]() 和H2O2)和相关基因表达的测定。
和H2O2)和相关基因表达的测定。
1.3.1 相对电导率和丙二醛含量的测定 相对电导率(Relative conductivity,RC)常用来评价细胞膜的损伤程度。用煮沸前和煮沸后的电导率(R1和R2分别表示煮沸前后溶液的电导率)来计算,公式为:RC(%)=R1/R2×100%。丙二醛(Malondialdehyde,MDA)含量采用硫代巴比妥酸显色法测定,采用公式6.45×(A532-A600)-0.56×A450计算各样品的MDA浓度,再根据样品质量计算MDA含量。
1.3.2 ROS含量的测定 超氧阴离子含量的测定:根据Elstner 等[23]和Jiang 等[24]对超氧阴离子测定原理的描述,采用羟胺氧化法进行![]() 含量的测定。植物中的
含量的测定。植物中的![]() 与盐酸羟胺反应生成
与盐酸羟胺反应生成![]() 在α-萘胺和对氨基苯磺酸的作用下,生成红色的偶氮化合物,在530 nm处测定其吸光值可以确定
在α-萘胺和对氨基苯磺酸的作用下,生成红色的偶氮化合物,在530 nm处测定其吸光值可以确定![]() 的浓度,根据样品质量计算样品中
的浓度,根据样品质量计算样品中![]() 的含量。
的含量。
过氧化氢含量的测定:植物中的H2O2与硫酸钛(或氯化钛)反应生成黄色的过氧化物-钛复合物沉淀,在H2SO4中进行溶解,在415 nm处有特征吸收峰,用分光光度计比色可测定H2O2含量[25]。
根据以上所描述的超氧阴离子和过氧化氢的测定原理和方法,采用南京建成生物工程研究所有限公司(苏州,中国)生产的科铭试剂盒进行![]() 和H2O2含量的测定[12,22]。
和H2O2含量的测定[12,22]。
1.3.3 萌发相关基因的表达分析 利用TRIzol法提取水稻幼芽总RNA,并测定RNA浓度,用M-MLV反转录酶(TaKaRaBioInc.,Otsu,Japan)进行反转录,形成cDNA。在实时荧光定量PCR仪(Eco TM 48,Illumina,Saffron Walden,UK)上进行qRT-PCR反应(Quantitative real-time PCR),总反应体系为20 μL,包括:10 μL 2×SYBR Premix Ex TaqTM(TaKaRa Bio),1.6 μL cDNA,0.8 μL引物和7.6 μL ddH2O。qRT-PCR反应程序为:95 ℃ 5 min;95 ℃ 5 s,60 ℃ 30 s,30个循环;95 ℃ 1 min,55 ℃ 30 s,95 ℃ 30 s。确定cDNA模板和引物没有基因组DNA污染后,以稳定表达的水稻看家基因β-actin作为内参,进行基因表达量的测定。每个处理设置3次生物学重复,每个模板2次技术重复,利用2-ΔΔCT的方法计算基因的相对表达量[26]。
ROS清除相关基因包括:OsCATB、OsAPX6、OsFe-SOD和OsCu/Zn-SOD;细胞死亡相关基因包括:OsBI1和OsKOD1;ABA应答基因有Salt和OsWsi18[12,22],用于qRT-PCR的基因及相关引物(5′-3′)如下:
OsACT1(内参,Os03g0718100)F:TTCCAGCCTT
CCTTCATA,R:AACGATGTTGCCATATAGAT;
OsBI1(Os02g0125300)F:CTACATCAAGCACGC
ACTC,R:ACCTCTTCTTCCTCTTCTTCTC;
OsKOD1(Os04g0507950)F:TCAAGCCATTCATC
TTCCAT,R:ATCAGCAACCTCGTCAAG;
Salt(Os01t0348900)F:CGAAATAATGTTCCATG
GTGTT,R:TGTACTACGGATCGGTGCAA;
OsWsi18(Os01g0705200)F:TGTGACTCGATCCA
GCGTAG,R:GTTCCTGCTGAGAAGCCATC;
OsCATB(Os06g0727200)F:GCTGGTGAGAGATACC
GGTCA,R:TCAACCCACCGCTGGAGA;
OsAPX6(Os12g0178100)F:CCCCAAGATCCCCA
TGATCTA,R:CCTCTGGCGGGCATTG;
OsFe-SOD(Os06g0143000)F:CGACGCCGAGGAAT
TTCTAG,R:AGGTGGTGTAAGTGTCTCTCATGC;
OsCu/Zn-SOD(Os06g0130900)F:TGTGACGGGA
CTTACTCCTGG,R:CACCCATTCGTAGTATCGCCA。
采用Excel 2013软件进行数据统计,数据绘图应用Origin 9.1绘图软件。利用SPSS 21.0(IBM Corp.,Armonk,NY)软件,基于单因素方差分析(ANOVA)和Duncan方法进行数据的显著性差异分析,显著性差异水平为P<0.05。
不同处理下,种子萌发试验结果如图1所示。非高温处理下,ABA浸种与否对种子萌发无明显影响。高温胁迫抑制了日本晴和黄华占的种子萌发,导致芽长和根长缩短。而ABA浸种能够促进高温胁迫下水稻种子的萌发和幼芽的生长。
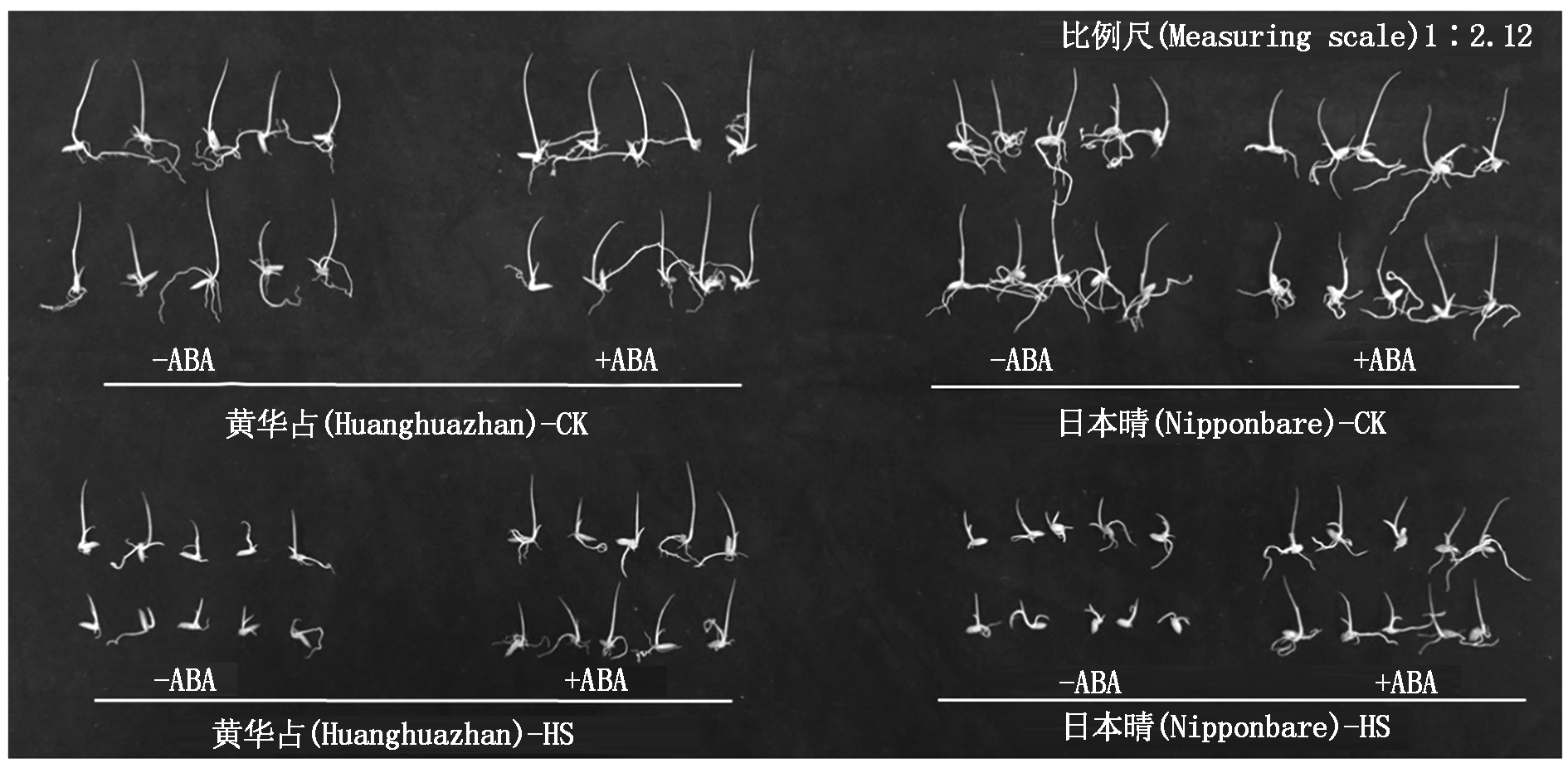
图1 ABA浸种对高温胁迫下水稻种子萌发的影响
Fig.1 Effect of seed soaking with ABA on rice seed germination under high temperature stress
发芽率统计试验结果表明(图2),ABA浸种在高温胁迫初期促进了水稻种子的萌发。非ABA浸种条件下,与CK-ABA相比,高温处理使日本晴在第2天和第3天的发芽率分别提高了11.60,7.60百分点,使黄华占在第2天,第3天的发芽率分别提高了14.00,4.00百分点。ABA浸种条件下,与CK+ABA相比,高温处理使日本晴在第2天,第3天的发芽率分别提高了7.60,9.20百分点,使黄华占在第2天,第3天的发芽率分别提高了14.40,6.80百分点。随着萌发时间的延长,不管ABA浸种与否,高温胁迫均抑制了水稻种子的萌发。在萌发的第7天,高温胁迫使ABA浸种和非ABA浸种的日本晴发芽率分别下降了12.00,22.80百分点,黄华占分别下降了10.40,20.00百分点。
对照条件下,ABA浸种的种子发芽率略低于非ABA浸种。而高温胁迫下,ABA浸种对水稻种子的萌发起到了一定的促进作用,且胁迫时间越长,促进作用越明显。从萌发的第5 天开始,高温胁迫条件下,ABA浸种提高了2个水稻品种的发芽率;在萌发的第6天,ABA浸种使日本晴和黄华占的发芽率分别提高了4.40,6.80百分点;第7天,ABA浸种使日本晴和黄华占的发芽率分别提高7.60,8.00百分点。
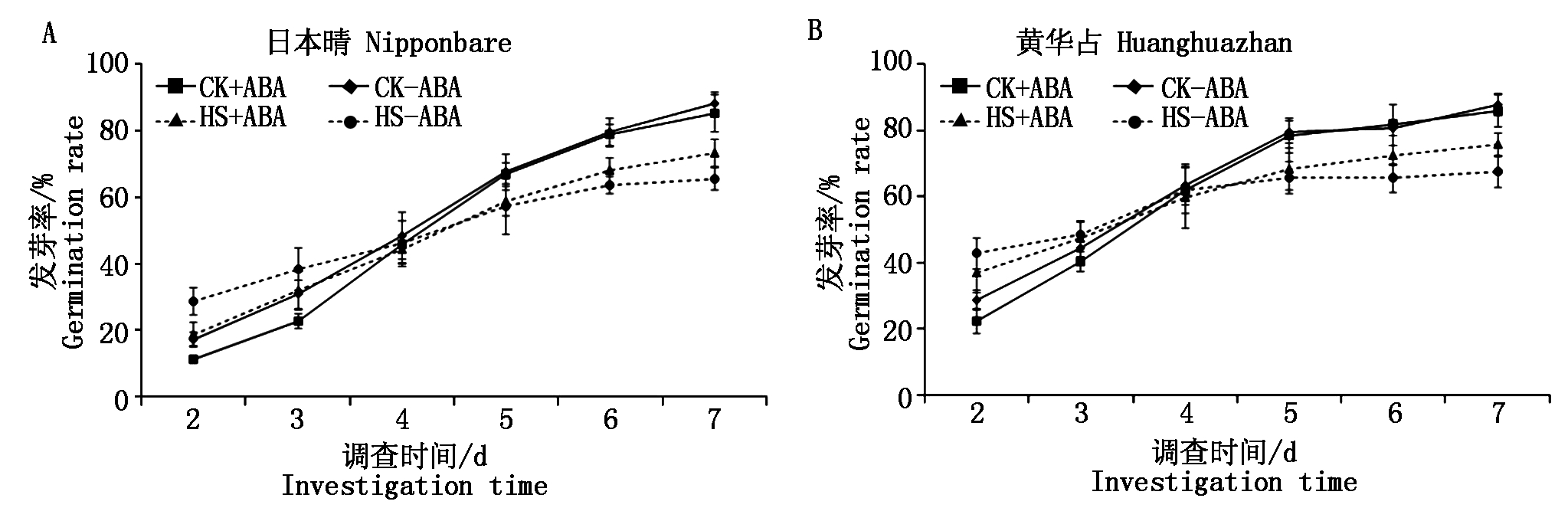
图2 ABA浸种对高温胁迫下水稻发芽率的影响
Fig.2 Effect of seed soaking with ABA on germination rate of rice under high temperature stress
ABA浸种促进了水稻芽和根的生长。试验结果表明(图3),对照条件下,ABA浸种对水稻幼芽和根的生长略有促进作用,ABA浸种显著提高了黄华占的主根长(P<0.05)。高温胁迫下,ABA浸种显著提高了水稻的芽长和主根长(P<0.05),黄华占的芽长增加更明显。高温胁迫下,ABA浸种使日本晴和黄华占的芽长分别提高了21.96%,38.92%;主根长分别提高了48.00%,63.82%。
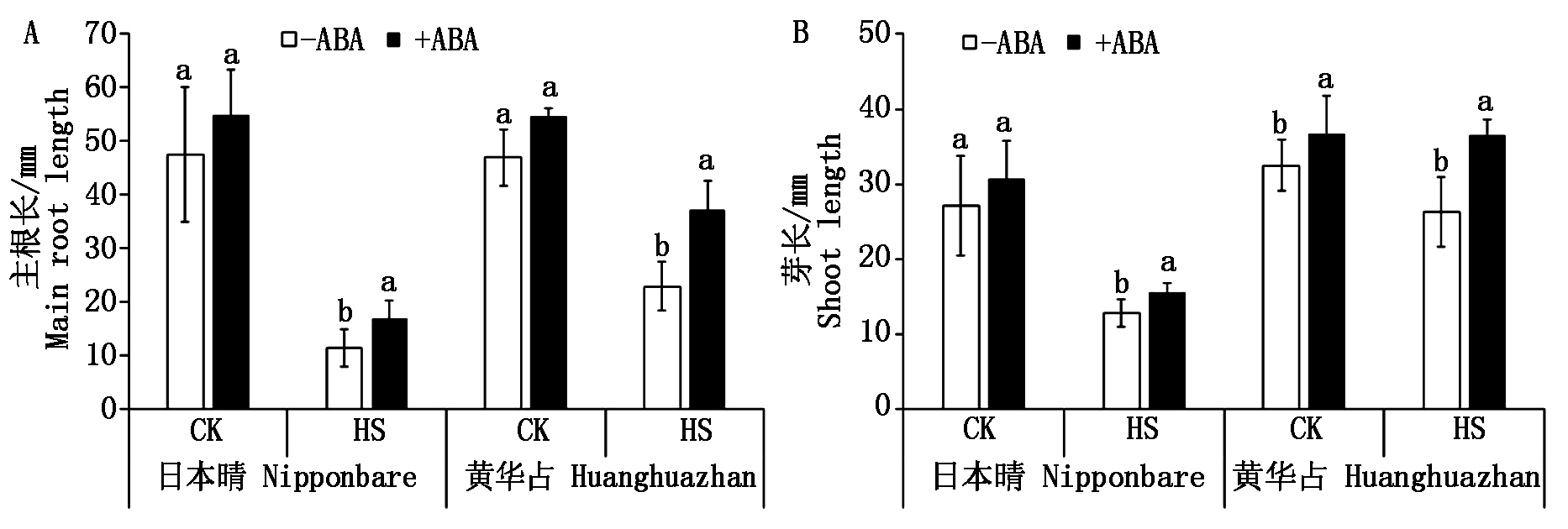
黑色与白色柱子上不同小写字母表示ABA浸种与非ABA浸种处理在0.05水平上差异显著。图4-7同。
Different small letters on the black or white columns indicate the significant difference was at P<0.05 level between ABA soaking and non-ABA soaking treatment. The same as Fig.4-7.
图3 ABA浸种对高温胁迫下水稻芽长和根长的影响
Fig.3 Effects of seed soaking with ABA on bud length and root length of rice under high temperature stress
高温胁迫抑制水稻生长发育的其中一个重要机制就是导致ROS过量积累。为了分析高温胁迫抑制种子萌发的机制及ABA浸种的效果,进一步调查了水稻幼芽中ROS的含量(图4)及ROS清除相关基因的表达量(图5)。由图4可知,对照条件下,2个品种在ABA浸种和非ABA浸种处理下幼芽的ROS含量无明显差异。不管ABA浸种与否,高温处理使2个品种幼芽的ROS含量均明显增加。高温胁迫下,与非ABA浸种相比,ABA浸种显著(P<0.05)降低了幼芽ROS的积累;高温处理下,ABA浸种下2个品种的![]() 含量(以鲜质量计)较非ABA浸种分别下降了26.02%,33.81%(图4-A);H2O2含量分别下降了25.62%,40.12%(图4-B)。
含量(以鲜质量计)较非ABA浸种分别下降了26.02%,33.81%(图4-A);H2O2含量分别下降了25.62%,40.12%(图4-B)。
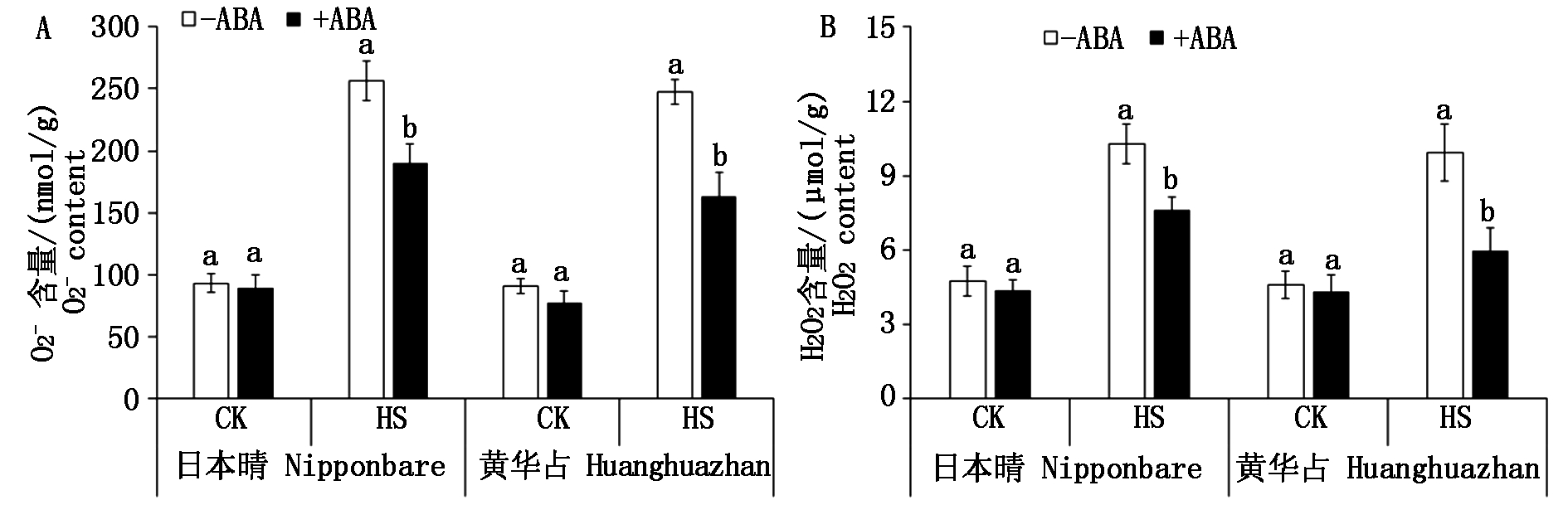
图4 ABA浸种对高温胁迫下水稻幼芽ROS积累的影响
Fig.4 Effects of seed soaking with ABA on ROS accumulation in rice bud under high temperature stress
研究表明,外源ABA预处理显著上调了多个ROS清除基因的表达,提升抗氧化酶活性,进而抑制ROS过量积累来提高水稻幼苗的耐碱性。在这些ROS清除基因中上调幅度较大的有OsCATB、OsAPX6、OsFe-SOD、OsCu/Zn-SOD,分别为过氧化氢酶(CAT)、抗坏血酸过氧化物酶(APX)和超氧化物歧化酶(SOD)的主要调控基因[22]。本试验继续分析高温胁迫下ABA浸种对以上4个ROS清除基因表达的影响。结果表明(图5),高温胁迫下,ABA浸种显著上调了4个ROS清除基因的表达(P<0.05),且OsFe-SOD和OsCu/Zn-SOD的上调幅度更大,暗示着ABA浸种提高了抗氧化酶CAT、APX和SOD的活性以清除过多的ROS。
研究表明,逆境胁迫下ROS的过量积累是导致水稻细胞损伤和幼苗萎蔫的主要原因[12],本试验进一步对高温胁迫下水稻幼芽的细胞损伤状况进行测定。由图6可知,高温胁迫条件下,ABA浸种处理使幼芽中细胞死亡抑制基因OsBI1的表达量显著上调(P<0.05),而细胞死亡基因OsKOD1表达下调,进而降低了高温胁迫导致的细胞过量死亡。高温胁迫下,与非ABA浸种处理相比,ABA浸种使2个品种OsBI1的上调幅度分别为182.0%,156.0%,而细胞死亡基因OsKOD1则分别下调了33.5%,51.3%。
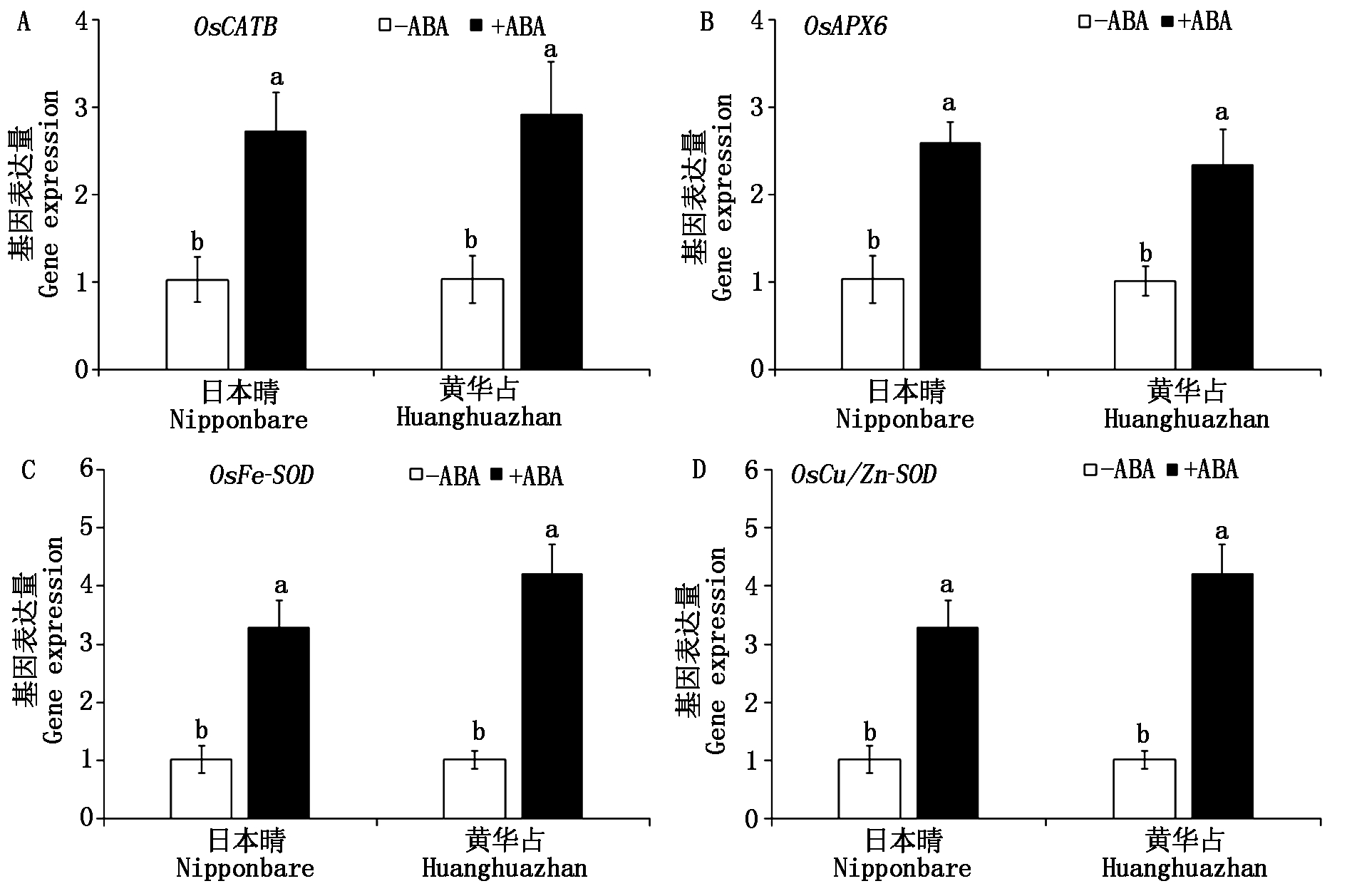
图5 ABA浸种对高温胁迫下水稻幼芽ROS清除相关基因的影响
Fig.5 Effects of seed soaking with ABA on expression of ROS-scavenging related genes under high temperature stress
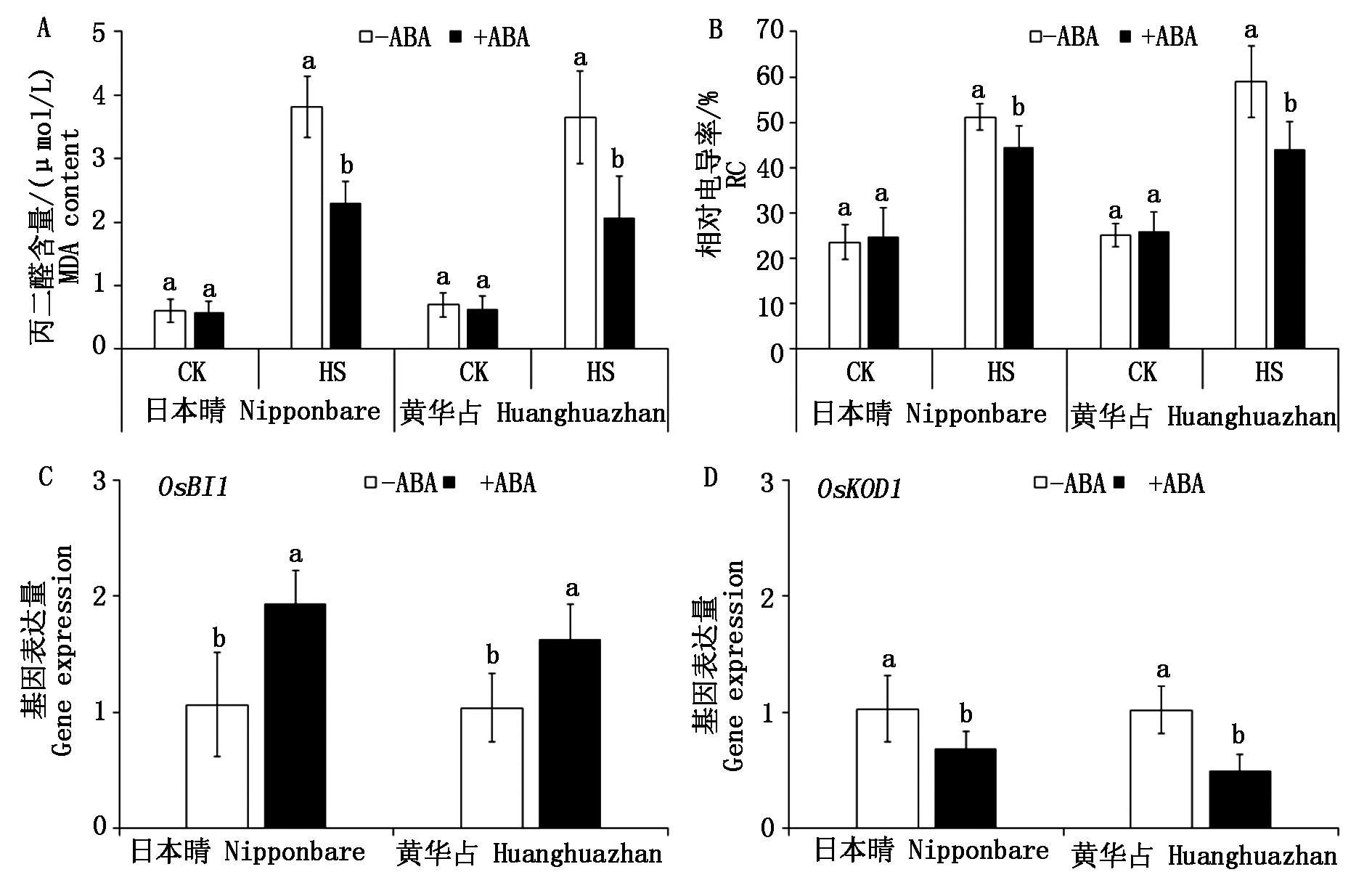
图6 ABA浸种对高温胁迫下水稻幼芽质膜损伤和细胞死亡基因表达的影响
Fig.6 Effects of seed soaking with ABA on the membrane injury and expression of cell death related genes in rice bud under high temperature stress
为进一步探究高温环境下ABA浸种预处理促进水稻种子萌发的机制,对种子萌发过程中的相关指标进行相关性分析。由表1可知,非ABA浸种条件下,发芽率与![]() 和MDA含量呈极显著负相关(P<0.01),且主根长与H2O2呈显著负相关(P<0.05),说明ROS过量积累是高温胁迫抑制水稻种子萌发的重要机制。ABA浸种条件下(表2),发芽率与
和MDA含量呈极显著负相关(P<0.01),且主根长与H2O2呈显著负相关(P<0.05),说明ROS过量积累是高温胁迫抑制水稻种子萌发的重要机制。ABA浸种条件下(表2),发芽率与![]() 含量和RC均呈极显著(P<0.01)负相关,且水稻种子的芽长和主根长与
含量和RC均呈极显著(P<0.01)负相关,且水稻种子的芽长和主根长与![]() 和H2O2的含量均呈显著(P<0.05)或极显著(P<0.01)负相关,与MDA和RC也呈负相关关系,但未达显著差异水平。
和H2O2的含量均呈显著(P<0.05)或极显著(P<0.01)负相关,与MDA和RC也呈负相关关系,但未达显著差异水平。
进一步分析了2个ABA应答基因的表达变化,结果如图7所示。高温胁迫下,ABA浸种处理下水稻幼芽内ABA应答基因显著(P<0.05)上调,说明ABA浸种激活了水稻内的ABA信号通路,使之在抵抗高温胁迫中发挥作用。高温胁迫下,与非ABA浸种处理相比,ABA浸种使日本晴和黄华占幼芽内Salt基因相对表达量分别提高了4.84,5.46倍(图7-A),OsWsi18分别提高了5.87,7.06倍(图7-B)。
表1 高温胁迫下非ABA浸种处理下水稻种子萌发进程中各指标的相关性分析
Tab.1 Correlation analysis of various indexes in rice seed germination process of non-ABA soaking treatment under high temperature stress condition
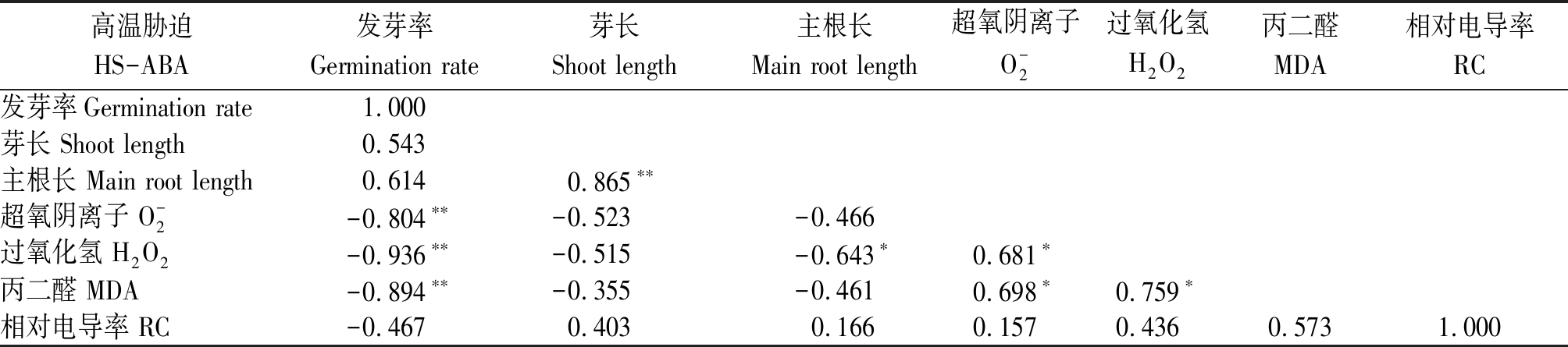
高温胁迫HS-ABA发芽率Germination rate芽长Shoot length主根长Main root length超氧阴离子O-2过氧化氢H2O2丙二醛MDA相对电导率RC发芽率Germination rate1.000芽长 Shoot length0.543主根长 Main root length0.6140.865∗∗超氧阴离子 O-2-0.804∗∗-0.523-0.466过氧化氢 H2O2-0.936∗∗-0.515-0.643∗0.681∗丙二醛 MDA-0.894∗∗-0.355-0.4610.698∗0.759∗相对电导率 RC-0.4670.4030.1660.1570.4360.5731.000
注:表中数值为相关系数(R2);**. 在0.01水平上显著相关;*. 在0.05水平上显著相关。表2同。
Note:The correlation coefficient (R2) was showed in the table; **. Significant difference at P<0.01 level;*. Significant difference at P<0.05 level. The same as Tab.2.
表2 高温胁迫下ABA浸种处理下水稻种子萌发进程中各指标的相关性分析
Tab.2 Correlation analysis of various indexes in rice seed germination process of ABA soaking treatment under high temperature stress condition
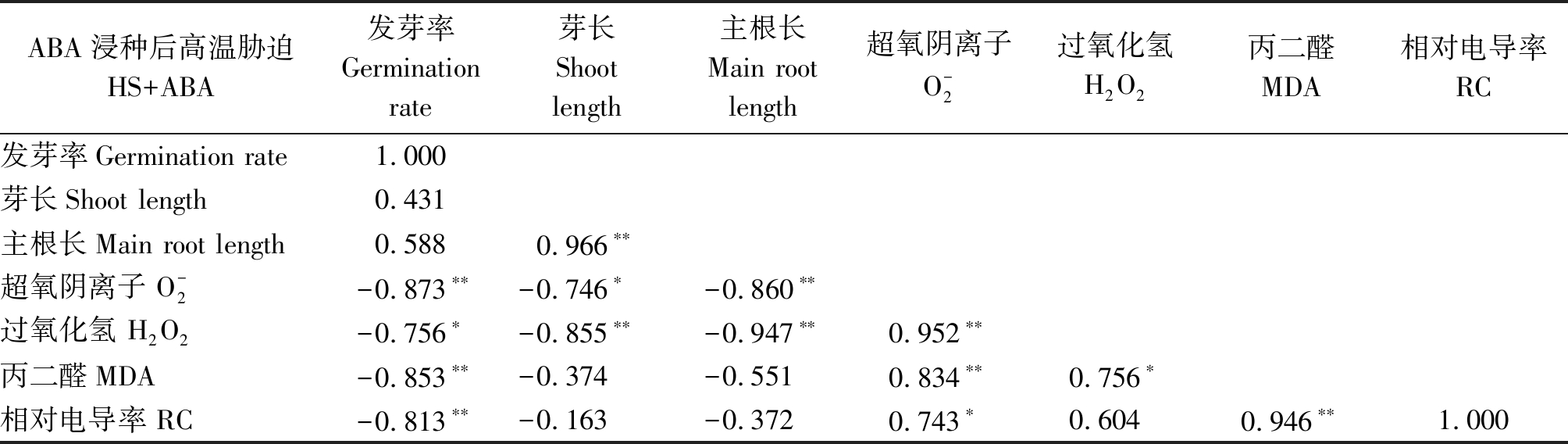
ABA浸种后高温胁迫HS+ABA发芽率Germination rate芽长Shoot length主根长Main root length超氧阴离子O-2过氧化氢H2O2丙二醛MDA相对电导率RC发芽率Germination rate1.000芽长Shoot length0.431主根长Main root length0.5880.966∗∗超氧阴离子 O-2-0.873∗∗-0.746∗-0.860∗∗过氧化氢 H2O2-0.756∗-0.855∗∗-0.947∗∗0.952∗∗丙二醛MDA-0.853∗∗-0.374-0.5510.834∗∗0.756∗相对电导率 RC-0.813∗∗-0.163-0.3720.743∗0.6040.946∗∗1.000
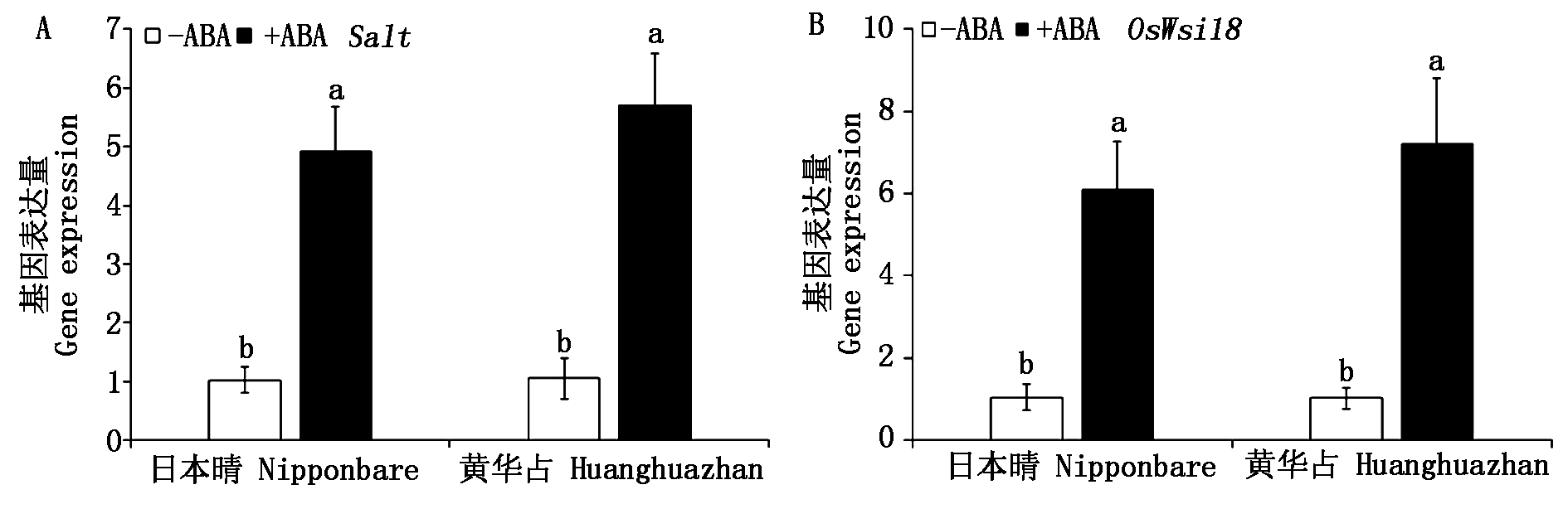
图7 高温胁迫下水稻幼芽ABA应答基因的表达量
Fig.7 Expression of ABA responsive genes in rice bud under high temperature stress
良好的种子萌发率是植物适应环境的先决条件。一般来说,水稻在种子萌发期对高温胁迫具有较强的抵抗力,这是因为诱导了种子中小热激蛋白的表达,进而能够帮助种子抵御高温;但超过35 ℃时水稻种子的萌发受阻,种子丧失活力,发芽率降低[27]。本研究结果表明,在萌发初期(前3 d),水稻种子在30 ℃的环境和40 ℃的高温环境下均能够正常萌发,且高温处理对种子萌发具有促进作用,发芽率略高于对照处理,这可能是由于种子经24 h浸泡后,含水量增加,能够在短期内抵抗高温的缘故,这与前人研究结果基本一致[28]。在萌发初期,对照条件和高温胁迫下,ABA浸种对种子萌发有抑制作用,2个品种的发芽率略低于非ABA浸种处理。随着萌发进程的推进,不管ABA浸种与否,与对照相比,高温胁迫均抑制了水稻种子的萌发;在萌发的第7 天,2个品种的发芽率在高温处理下较对照均有不同程度的下降,且芽长和主根长显著(P<0.05)低于对照处理,这与多数研究结果基本一致[29-31]。不管ABA浸种与否,在对照条件下,品种黄华占的芽长和主根长高于日本晴;且在高温胁迫下的芽长和主根长的下降幅度以及幼芽内ROS积累和质膜损伤程度均低于日本晴,说明黄华占在种子萌发期对高温的抗性略高于日本晴。
ABA不仅参与调控植物的多种生长发育进程,且在植物应对盐、碱、低温和高温等非生物胁迫和多种生物胁迫中发挥重要作用[15,32]。ABA调控植物抗逆性的一种重要机制就是产生诱抗效应,通过浸种和浸根等多种方式赋予植物抵抗逆境的潜在能力[18,22-33]。研究表明,水稻种子或幼苗经ABA预处理后能够提高盐胁迫下幼苗的生长发育和最终产量[34-35];水稻幼苗经外源ABA浸根预处理后,能够提高抗氧化清除能力,抑制ROS的过量积累,进而提高对碱胁迫的抗性和苏打盐碱水田中的产量[17-18,22]。在高温胁迫下,外源ABA能够通过多种途径缓解高温胁迫对水稻的伤害,包括调控能量平衡、ROS积累和糖代谢等途径,促进水稻生长发育和产量形成[2,21,36]。本试验结果表明,种子萌发初期,在对照和高温处理下,外源ABA浸种较非ABA浸种在一定程度上抑制了种子的萌发。在长期高温胁迫下,外源ABA浸种加快了水稻种子的萌发速度,促进了幼芽和幼根的生长,缓解了长期极端高温胁迫对水稻种子幼芽生长的抑制作用。对其内在机制进一步研究发现,高温胁迫下,ABA浸种显著(P<0.05)上调了ABA应答基因Salt和OsWsi18的表达量,说明ABA信号途径被激活,进一步提升了高温胁迫下水稻幼芽抗氧化清除基因的转录表达,降低ROS的含量,进而缓解了水稻幼芽的质膜损伤和细胞过量死亡,促进幼芽的生长。
逆境胁迫会破坏植物体内ROS产生和清除的平衡体系,破坏抗氧化防御系统,导致ROS过量积累,将进一步导致细胞结构、DNA、RNA和蛋白质等结构的损伤[37];在水稻中,ROS的过量积累还是导致根系损伤和细胞死亡的重要原因[12]。高温胁迫导致植物中ROS过量积累,并进一步加剧了膜质过氧化作用,并影响了花粉发育、淀粉合成和光合作用等多个生理代谢过程,最终影响产量[38-39]。ABA提高植物抗逆性的一个重要机制就是提升逆境胁迫下植物的抗氧化防御能力,外源ABA预处理能够通过提高抗氧化酶活性、降低质膜损伤和调节气孔关闭等途径来提高水稻对干旱和低温胁迫的抗性[40-41]。研究表明,ABA能够提高外源二氯百草枯对水稻产生的氧化胁迫,降低ROS含量和质膜损伤,进而缓解幼苗萎蔫和死亡[22]。在本试验中,高温胁迫下,不管ABA浸种与否,水稻种子的发芽率与![]() 和H2O2含量均呈极显著(P<0.05)或显著(P<0.01)负相关关系,说明ROS的过量积累是高温胁迫抑制种子萌发的关键因素。而ABA浸种条件下,芽长和主根长与
和H2O2含量均呈极显著(P<0.05)或显著(P<0.01)负相关关系,说明ROS的过量积累是高温胁迫抑制种子萌发的关键因素。而ABA浸种条件下,芽长和主根长与![]() 和H2O2含量也显著(P<0.05)或极显著(P<0.01)负相关,且ABA浸种后显著(P<0.05)降低了高温胁迫下水稻幼芽内ROS含量和质膜损伤程度,提升抗氧化清除能力,并进一步下调了细胞死亡基因OsKOD1的表达和上调了细胞死亡抑制基因OsBI1的表达。以上结果进一步表明,高温胁迫下,ABA浸种可以通过降低ROS的过量积累和缓解质膜损伤来促进水稻种子的萌发和幼芽的生长。
和H2O2含量也显著(P<0.05)或极显著(P<0.01)负相关,且ABA浸种后显著(P<0.05)降低了高温胁迫下水稻幼芽内ROS含量和质膜损伤程度,提升抗氧化清除能力,并进一步下调了细胞死亡基因OsKOD1的表达和上调了细胞死亡抑制基因OsBI1的表达。以上结果进一步表明,高温胁迫下,ABA浸种可以通过降低ROS的过量积累和缓解质膜损伤来促进水稻种子的萌发和幼芽的生长。
综上所述,本研究探究了高温胁迫下ABA浸种对水稻种子萌发的影响,进一步验证了外源ABA对水稻抗逆性的诱抗效应。水稻种子在萌发初期,高温处理能够短暂的促进种子萌发;此后,高温处理导致幼芽内ROS过量积累,并进一步导致细胞损伤,进而抑制了幼芽的生长。种子在萌发前利用外源ABA浸种能够起到提高抗氧化清除能力的作用,降低由极端高温胁迫而引起的ROS过量积累,从而缓解细胞损伤,促进高温胁迫下水稻幼芽及幼根的生长。
[1] 段骅,杨建昌. 高温对水稻的影响及其机制的研究进展[J].中国水稻科学,2012,26(4):393-400. doi:10.3969/j.issn.1001-7216.2012.04.002.
Duan H,Yang J C. Research advances in the effect of high temperature on rice and its mechanism[J].Chinese Journal of Rice Science,2012,26(4):393-400.
[2] Li G Y,Zhang C X,Zhang G H,Fu W M,Feng B H,Chen T T,Peng S B,Tao L X,Fu G F. Abscisic acid negatively modulates heat tolerance in rolled leaf rice by increasing leaf temperature and regulating energy homeostasis[J].Rice,2020,13(1):18.doi:10.1186/s12284-020-00379-3.
[3] 冯活仪,江浩林,王孟,唐湘如,段美洋,潘圣刚,田华,王树丽,莫钊文. 不同香稻品种苗期耐高温的形态生理响应[J].中国水稻科学,2019,33(1):68-74.doi:10.16819/j.1001-7216.2019.8022.
Feng H Y,Jiang H L,Wang M,Tang X R,Duan M Y,Pan S G,Tian H,Wang S L,Mo Z W. Morphophysiological responses of different scented rice varieties to high temperature at seedling stage[J]. Chinese Journal of Rice Science,2019,33(1):68-74.
[4] 杨卫丽,黄福灯,曹珍珍,雷炳婷,胡东维,程方民. 高温胁迫对水稻光合PSⅡ系统伤害及其与叶绿体D1蛋白间关系[J].作物学报,2013,39(6):1060-1068. doi:10.3724/SP.J.1006.2013.01060.
Yang W L,Huang F D,Cao Z Z,Lei B T,Hu D W,Cheng F M. Effects of high temperature stress on PSⅡ function and its relation to D1 protein in chloroplast thylakoid in rice flag leaves[J].Acta Agronomica Sinica,2013,39(6):1060-1068.
[5] Fan M H,Sun X,Xu N J,Liao Z,Li Y H,Wang J X,Fan Y P,Cui D L,Li P,Miao Z L. Integration of deep transcriptome and proteome analyses of salicylic acid regulation high temperature stress in Ulva prolifera[J]. Scientific Reports,2017,7(1):11502. doi:10.1038/s41598-017-11449-w.
[6] Feng B H,Zhang C X,Chen T T,Zhang X F,Tao L X,Fu G F. Salicylic acid reverses pollen abortion of rice caused by heat stress[J]. BMC Plant Biology,2018,18(1):245. doi:10.1186/s12870-018-1472-5.
[7] Wang Y L,Wang L,Zhou J X,Hu S B,Chen H Z,Xiang J,Zhang Y K,Zeng Y J,Shi Q H,Zhu D F,Zhang Y P. Research progress on heat stress of rice at flowering stage[J]. Rice Science,2019,26(1):1-10. doi:10.1016/j.rsci.2018.06.009.
[8] Fu G F,Feng B H,Zhang C X,Yang Y J,Yang X Q,Chen T T,Zhao X,Zhang X F,Jin Q Y,Tao L X. Heat stress is more damaging to superior spikelets than inferiors of rice (Oryza sativa L.) due to their different organ temperatures[J]. Frontiers in Plant Science,2016,7:1637. doi:10.3389/fpls.2016.01637.
[9] Zhang C X,Fu G F,Yang X Q,Yang Y J,Zhao X,Chen T T,Zhang X F,Jin Q Y,Tao L X. Heat stress effects are stronger on spikelets than on flag leaves in rice due to differences in dissipation capacity[J]. Journal of Agronomy and Crop Science,2016,202(5):394-408. doi:10.1111/jac.12138.
[10] Zhang C X,Feng B H,Chen T T,Fu W M,Li H B,Li G Y,Jin Q Y,Tao L X,Fu G F. Heat stress-reduced kernel weight in rice at anthesis is associated with impaired source-sink relationship and sugars allocation[J]. Environmental and Experimental Botany,2018,155:718-733. doi:10.1016/j.envexpbot.2018.08.021.
[11] Coast O,Murdoch A J,Ellis R H,Hay F R,Jagadish K S V. Resilience of rice (Oryza spp.) pollen germination and tube growth to temperature stress[J].Plant Cell and Environment,2016,39(1):26-37. doi:10.1111/pce.12475.
[12] Zhang H,Liu X L,Zhang R X,Yuan H Y,Wang M M,Yang H Y,Ma H Y,Liu D,Jiang C J,Liang Z W. Root damage under alkaline stress is associated with reactive oxygen species accumulation in rice (Oryza sativa L.)[J]. Frontiers in Plant Science,2017,8:1580. doi:10.3389/fpls.2017.01580.
[13] Matsui T,Kobayasi K,Kagata H,Horie T. Correlation between viability of pollination and length of basal dehiscence of the theca in rice under a hot-and-humid condition[J]. Plant Production Science,2005,8(2):109-114. doi:10.1626/pps.8.109.
[14] Zhao Q,Zhou L J,Liu J C,Du X X,Asad M A U,Huang F D,Pan G,Cheng F M. Relationship of ROS accumulation and superoxide dismutase isozymes in developing anther with floret fertility of rice under heat stress[J]. Plant Physiology and Biochemistry,2018,122:90-101. doi:10.1016/j.plaphy.2017.11.009.
[15] Dar N A,Amin I,Wani W,Wani S A,Shikari A B,Wani S H,Masoodi K Z. Abscisic acid:A key regulator of abiotic stress tolerance in plants[J]. Plant Gene,2017,11:106-111. doi:10.1016/j.plgene.2017.07.003.
[16] Savvides A,Ali S,Tester M,Fotopoulos V. Chemical priming of plants against multiple abiotic stresses:Mission possible?[J]. Trends in Plant Science,2016,21(4):329-340.doi:10.1016/j.tplants.2015.11.003.
[17] Wei L X,Lü B S,Wang M M,Ma H Y,Yang H Y,Liu X L,Jiang C J,Liang Z W. Priming effect of abscisic acid on alkaline stress tolerance in rice (Oryza sativa L.) seedlings[J]. Plant Physiology and Biochemistry,2015,90:50-57. doi:10.1016/j.plaphy.2015.03.002.
[18] Wei L X,Lü B S,Li X W,Wang M M,Ma H Y,Yang H Y,Yang R F,Piao Z Z,Wang Z H,Lou J H,Jiang C J,Liang Z W. Priming of rice (Oryza sativa L.) seedlings with abscisic acid enhances seedling survival,plant growth,and grain yield in saline-alkaline paddy fields[J]. Field Crops Research,2017,203:86-93. doi:10.1016/j.fcr.2016.12.024.
[19] Hu X J,Chen D D,Mclntyre C L,Dreccer M F,Zhang Z B,Drenth J,Kalaipandian S,Chang H P,Xue G P. Heat shock factor C2a serves as a proactive mechanism for heat protection in developing grains in wheat via an ABA-mediated regulatory pathway[J].Plant,Cell & Environment,2018,41(1):79-98.doi:10.1111/pce.12957.
[20] 王强,陈雷,张晓丽,唐茂艳,吕荣华,陶伟,梁天锋.化学调控对水稻高温热害的缓解作用研究[J]. 中国稻米,2015,21(4):80-82.doi:10.3969/j.issn.1006-8082.2015.04.017.
Wang Q,Chen L,Zhang X L,Tang M Y,Lü R H,Tao W,Liang T F. Alleviative effect of chemical regulators on rice in high temperature stress[J].China Rice,2015,21(4):80-82.
[21] Rezaul I M,Feng B H,Chen T T,Fu W M,Zhang C X,Tao L X,Fu G F. Abscisic acid prevents pollen abortion under high-temperature stress by mediating sugar metabolism in rice spikelets[J].Physiologia Plantarum,2019,165(3):644-663. doi:10.1111/ppl.12759.
[22] Liu X L,Zhang H,Jin Y Y,Wang M M,Yang H Y,Ma H Y,Jiang C J,Liang Z W. Abscisic acid primes rice seedlings for enhanced tolerance to alkaline stress by upregulating antioxidant defense and stress tolerance-related genes[J]. Plant and Soil,2019,438(1/2):39-55. doi:10.1007/s11104-019-03992-4.
[23] Elstner E F,Heupel A. Inhibition of nitrite formation from hydroxylammoniumchloride:A simple assay for superoxide dismutase[J]. Analytical Biochemistry,1976,70(2):616-620. doi:10.1016/0003-2697(76)90488-7.
[24] Jiang M Y,Zhang J H. Effect of abscisic acid on active oxygen species,antioxidative defence system and oxidative damage in leaves of maize seedlings[J]. Plant and Cell Physiology,2001,42(11):1265-1273. doi:10.1093/pcp/pce162.
[25] Brennan T,Frenkel C. Involvement of hydrogen peroxide in the regulation of senescence in pear[J]. Plant Physiology,1977,59(3):411-416. doi:10.1104/pp.59.3.411.
[26] Livak K J,Schmittgen T D. Analysis of relative gene expression data using Real-time quantitative PCR and the 2-ΔΔCT method[J]. Methods,2001,25(4):402-408. doi:10.1006/meth.2001.1262.
[27] 唐婷,陈艳艳,杨洋,杨远柱,孟桂元,周静.水稻耐高温性研究进展[J].分子植物育种,2017,15(9):3694-3700.doi:10.13271/j.mpb.015.003694.
Tang T,Chen Y Y,Yang Y,Yang Y Z,Meng G Y,Zhou J. Research progress of rice resistance to high temperature[J].Molecular Plant Breeding,2017,15(9):3694-3700.
[28] 张克勤,冯玉强,吴荣梁,李春生,孔宪琴,鄂志国. 不同水稻品种种子在高温高湿条件下的发芽率变化[J]. 中国稻米,2011,17(6):49-52. doi:10.3969/j.issn.1006-8082.2011.06.015.
Zhang K Q,Feng Y Q,Wu R L,Li C S,Kong X Q,E Z G. Germination rate of different rice varieties under high temperature and humidity[J]. China Rice,2011,17(6):49-52.
[29] 叶世青. 高温处理对杂交水稻种子发芽率的影响[J]. 杂交水稻,2011,26(6):34-36.doi:10.3969/j.issn.1005-3956.2011.06.012.
Ye S Q. Effects of high temperature on germination of hybrid rice seeds[J]. Hybrid Rice,2011,26(6):34-36.
[30] 叶世青. 高温处理对不同状态水稻种子发芽率的影响[J]. 中国稻米,2017,23(6):47-52. doi:10.3969/j.issn.1006-8082.2017.06.009.
Ye S Q. Effects of high temperature on germination rate of rice seed[J]. China Rice, 2017,23(6):47-52.
[31] Liu S J,Xu H H,Wang W Q,Li N,Wang W P,Møller I M,Song S Q. A proteomic analysis of rice seed germination as affected by high temperature and ABA treatment[J]. Physiologia Plantarum,2015,154(1):142-161. doi:10.1111/ppl.12292.
[32] Sah S K,Reddy K R,Li X J. Abscisic acid and abiotic stress tolerance in crop plants[J].Frontiers in Plant Science,2016,7:571.doi:10.3389/fpls.2016.00571.
[33] Jones A M. A new look at stress:abscisic acid patterns and dynamics a thigh-resolution[J].New Phytologist,2016,210(1):38-44. doi:10.1111/nph.13552.
[34] Gurmani A R,Bano A,Salim M. Effect of growth regulators on growth,yield and ions accumulation of rice (Oryza sativa L.) under salt stress[J].Pakistan Journal of Botany,2006,38(5):1415-1424. doi:10.1110/ps.062292106.
[35] Li X J,Yang M F,Chen H,Qu L Q,Chen F,Shen S H. Abscisic acid pretreatment enhances salt tolerance of rice seedlings:proteomic evidence[J]. Biochimica et Biophysica Acta,2010,1804(4):929-940. doi:10.1016/j.bbapap.2010.01.004.
[36] Li N,Euring D,Cha J Y,Lin Z,Lu M Z,Huang L J,Kim W Y. Plant Hormone-mediated regulation of heat tolerance in response to global climate change[J]. Frontiers in Plant Science,2021,11:627969. doi:10.3389/fpls.2020.627969.
[37] Choudhury F K,Rivero R M,Blumwald E,Mittler R. Reactive oxygen species,abiotic stress and stress combination[J]. The Plant Journal,2017,90(5):856-867. doi:10.1111/tpj.13299.
[38] Suriyasak C,Harano K,Tanamachi K,Matsuo K,Tamada A,Iwaya-lnoue M,Ishibashi Y. Reactive oxygen species induced by heat stress during grain filling of rice (Oryza sativa L.) are involved in occurrence of grain chalkiness[J]. Journal of Plant Physiology,2017,216:52-57. doi:10.1016/j.jplph.2017.05.015.
[39] 尤翠翠,何海兵,王华运,吴汉,汪跃君,尚蓉霞,张津,时强强,武立权. 花期高温对水稻穗部的伤害机理及氮素调控效应研究[C]//安徽水稻与稻作技术论文汇编.合肥:中国作物学会,2017:124-125.
You C C,He H B,Wang H Y,Wu H,Wang Y J,Shang R X,Zhang J,Shi Q Q,Wu L Q. Study on damage mechanism and nitrogen regulation effect of high temperature in flowering period on panicle of rice[C]//Compilation of papers on Anhui rice and rice cropping technology. Hefei: Chinese society of crop sciences,2017:124-125.
[40] Wang G J,Miao W,Wang J Y,Ma D R,Li J Q,Chen W F. Effects of exogenous abscisic acid on antioxidant system in weedy and cultivated rice with different chilling sensitivity under chilling stress[J].Journal of Agronomy and Crop Science,2013,199(3):200-208.doi:10.1111/jac.12004.
[41] 郭贵华,刘海艳,李刚华,刘明,李岩,王绍华,刘正辉,唐设,丁艳锋. ABA缓解水稻孕穗期干旱胁迫生理特性的分析[J].中国农业科学,2014,47(22):4380-4391. doi:10.3864/j.issn.0578-1752.2014.22.004.
Guo G H,Liu H Y,Li G H,Liu M,Li Y,Wang S H,Liu Z H,Tang S,Ding Y F. Analysis of physiological characteristics about ABA alleviating rice booting stage drought stress[J].Scientia Agricultura Sinica,2014,47(22):4380-4391.