设施蔬菜栽培是现代农业生产的重要组成部分,能够显著提高自然资源利用效率,增加农民经济收益[1]。中国是全世界最大的设施蔬菜生产国,且种植规模仍以每年10%左右的速度增长。2014 年全国设施蔬菜种植面积达到386×104 hm2,产值超7 000亿元,从业人员4 000万以上[2]。根据农业农村部《全国设施蔬菜重点区域发展规划(2015-2020年)》,到2020年,全国将新增设施蔬菜生产面积7.0×104 hm2,总产值超过10 000亿元,其收入占农民人均纯收入10%以上。在近年来农业供给侧结构性改革的大背景下,促进设施蔬菜行业的提质增效和健康发展对于解决三农问题、促进返乡就业和实现乡村振兴的战略目标具有重要意义。然而就整个设施蔬菜产业链而言,在生产环节由于长期集约化种植所带来的连作障碍问题依然是限制我国设施蔬菜产业可持续发展的重要瓶颈之一[3]。因此,迫切需要阐明设施蔬菜连作障碍形成机理。
集约化设施蔬菜栽培呈现土壤耕作频繁、复种指数高和水、肥供应量大的显著特点,菜农受利益驱使,长期连作种植导致土壤恶化、病虫害猖獗、经济产量降低和品质下降等严重问题。健康的土壤环境是作物高产高效和农业可持续发展的基础,尽管不同作物(品种)或地区之间的连作障碍机理可能存在差别,但主要源自土壤[4]。土壤是组分多样化、具有高度异质性和复杂性且易于扰动的生态系统,长期连作常常导致土壤性质劣变,并对作物生长发育形成负反馈调节[5-6],阐明设施蔬菜连作条件下土壤障碍因子(类型)是理清连作障碍机理并发展形成高效的连作土壤障碍因子消减技术的重要前提。早前的学者也从设施连作土壤理化和生化性质变化角度得到了大量研究结论,诸如土壤物理结构恶化(土壤板结和团聚体稳定性降低)、养分不均衡吸收和作物根区养分亏缺、土壤酸化和次生盐渍化、土壤酶活性衰减、化感(自毒)作用、土传致病菌滋生和根结线虫富集等[7-8]。
微生物是土壤生态系统的重要成员,其在遗传、结构和功能上具有高度的多样性,有效驱动着有机质分解、养分循环、污染物降解和能量流动等一系列土壤生物学过程,同时在抑制土传病害和维持作物健康方面发挥重要作用。随着分子生物学技术的进步和组学技术的应用,土壤微生物及其群落组成和结构在连作种植条件下的行为变化特征越来越受到研究者的关注。研究业已证明,长期连作能够显著改变土壤中微生物数量和群落结构,表现为降低土壤微生物多样性,并随着连作年限延长,土壤微生物群落结构显著改变,从“细菌型”向“真菌型”转变,土传病害显著增加[9-15]。进一步研究显示,长期连作导致土壤微生物群落结构受到破坏,种间互作呈现无序和弱化的状态,且有益微生物或具有潜在生防能力的微生物丰度降低,对土传致病菌的竞争能力下降[16-24]。然而,微生物受作物种类(品种)、土壤类型(质地)、地理环境和气候、耕作和栽培条件、养分供应等各种因素影响较大,不同地区间土壤微生物种类、数量、多样性和组成结构等存在明显差异,因而其对连作种植的响应特征可能存在差别。豫北地区是河南省划定的蔬菜种植产业优势区,但区域内设施蔬菜连作障碍的土壤微生态学机理迄今为止鲜有报道。鉴于此,以黄瓜这一传统的设施蔬菜主栽作物为研究对象,通过采集具有不同黄瓜连作年限的土壤样品,结合Real-time PCR和高通量测序等技术手段,研究了设施黄瓜连作对土壤真菌数量和群落结构的影响,旨在为阐明区域内设施蔬菜连作障碍机理和发展相关的连作土壤障碍因子消减技术提供帮助。
1 材料和方法
1.1 试验区概况
研究区域为河南省新乡市牧野区朱庄屯村(地理坐标35°21′12″ N,113°55′2″ E,平均海拔约56 m),供试土壤为壤质潮土。该区地处黄河中下游,属于暖温带大陆性季风气候,四季分明,雨热同季,降水集中,年均气温14 ℃,全年无霜期220 d,年均降水量573.4 mm,水资源丰富,土层深厚,土壤肥沃。设施蔬菜方面,主要栽培黄瓜、番茄、辣椒和茄子等,种植结构相对单一,且大多处于长期连续种植状态,连作障碍问题突出,枯萎病、黄萎病和青枯病等土传病害较为普遍。
1.2 试验设计和样品采集
在试验区内选择连作年限分别为1,5,10,15,20 a的黄瓜种植大棚各3个,用土钻按五点取样法在每个棚内采集混合样品1个,采样深度为0~20 cm,即每个连作年限处理下共得到了相当于3次重复的土壤样本,5个连作年限处理分别用CC1、CC5、CC10、CC15和CC20表示。CC1地块前茬作物为玉米,2017年春季开始转为温室黄瓜生产。土样采集在2017年秋季黄瓜收获时进行,以尽可能排除植株根系和其他一些农业管理措施对土壤样本的潜在影响。将采集的新鲜土样去除石块、动植物残体等杂质后,装入干净的密封袋,带回实验室过1 mm筛,将过筛后土壤分成3份,一份储存于-80 ℃冰箱内用于后续的微生物分子生物学分析,一份在室温下风干用于测定土壤常规理化性质,另一份立刻用于测定土壤矿质氮含量和相关生化性质。供试土壤理化和生化性质测定方法见Liu等[25]的报道。为最大限度地消除不同地块和年份间种植和管理措施对土壤取样和后续数据分析的影响,本研究中所有温室地块均来自相同的农民种植合作社,彼此相距较近,且一直采用当地统一的耕作种植和农艺管理方法,具体细节见文献[25]。
1.3 土壤真菌数量测定
按产品说明书上的步骤,使用PowerSoil DNA试剂盒提取微生物总DNA。采用1%琼脂糖凝胶电泳和分光光度法检测DNA的质量和纯度。将提取的DNA溶解在50 μL洗脱缓冲液中并于-20 ℃冰箱中保存备用。真菌PCR扩增采用18S rRNA基因V5-V7区特异性通用引物对SSU0817F/1196R[26]。荧光定量PCR采用ABI 7500 实时定量PCR仪进行,每个土样重复3次,扩增体系(20 μL)如下: 10.0 μL ChamQ SYBR Color qPCR Master Mix(2×),引物各0.8 μL(5 μmol/L),2 μL DNA模板,6.4 μL ddH2O。扩增条件为: 95 ℃ 5 min预变性;94 ℃ 5 s,55 ℃ 30 s,72 ℃ 40 s,30个循环。在每一循环退火阶段收集荧光,实时检测反应并且记录荧光信号变化,得出扩增产物熔解曲线。所得产物用1%琼脂糖凝胶电泳检测,扩增片段大小为425 bp,通过熔解曲线验证扩增专一性。扩增产物经纯化后并连接至pMD18-T克隆载体上,转化大肠杆菌,挑取转化后平板上的白色单克隆提取质粒,测序确定插入DNA片段是否正确。采取10倍梯度稀释标准质粒,用10-2~10-7稀释液制备标准曲线。确定阈值和基线,绘制标准曲线,横坐标为基因拷贝数的常用对数,纵坐标为荧光定量PCR测得的Ct值。PCR扩增效率93.96%,标准曲线R2为0.999 4。通过Ct值,计算待测样品中真菌数量,并进一步计算各土壤样品的真菌和细菌数量比值,细菌数量已在前期文献中报道[25],此处不再重复。
1.4 土壤真菌群落结构和生物信息学分析
采用高通量扩增子测序方法(Illumina Miseq PE300高通量测序平台)评估不同连作年限处理下土壤真菌的多样性和群落结构。以土壤微生物总DNA为模板进行PCR扩增,扩增引物对为SSU0817F/1196R[26]。PCR扩增体系(20 μL)为: 4 μL FastPfu Buffer(5×),2 μL dNTPs(2.5 mmol/L),0.4 μL FastPfu Polymerase,0.2 μL Bovine serum albumin,引物各0.8 μL(5 μmol/L),10 ng 模板DNA和11.2 μL ddH2O。PCR扩增反应条件为: 95 ℃预变性3 min;95 ℃变性30 s,55 ℃退火30 s,72 ℃延伸40 s,35个循环;最后72 ℃再延伸10 min。PCR产物经琼脂糖凝胶电泳验证,采用Agarose Gel DNA纯化试剂盒进行纯化,并用分光光度计测定浓度,最后将PCR产物等量混合,送上海美吉生物医药科技有限公司进行高通量测序。
将高通量测序下机原始序列进行双端序列拼接和质量过滤,使用QIIME软件分析[27]。对序列进行质控,删除尾部质量值20以下、长度小于200 bp的序列; 根据序列首尾两端的标签和引物区分样品,并调整序列方向,序列标签允许的错配数为0,最大引物错配数为2,将序列分配至不同处理样品;使用Uclust的方法按照97%的相似度进行可操作分类单元(Operational taxonomic units OTU)聚类,每个OTU中数量最多的序列被挑选为代表序列;为得到每个OTU对应的物种分类信息,采用RDP classifier贝叶斯算法对97%相似水平的OTU代表序列进行分类学比对,以Silva 128/18S_Eukaryota数据库为参考,对OTU代表序列进行比对分类鉴定,分类置信度阈值为70%。本研究15个土壤样本总计获得642 481条高质量序列,序列平均长度为401 bp;对所得到的高质量序列,按最小样本序列数抽平(每个土壤样本31 374条序列),用于后续的α多样性和β多样性分析。根据OTU列表中各样品物种丰度情况,应用软件MOTHUR计算菌群α多样性指数。使用QIIME软件,对Weighted的UniFrac距离矩阵进行UPGMA聚类分析。基于Bray-curtis距离算法的主坐标分析(Principal coordinates analysis,PCoA)用于比较不同处理土壤真菌群落结构差异,并利用相似度分析(Analysis of similarities,ANOSIM)检验处理间真菌群落结构的差异显著性。对少数平均丰度较高且在Silva数据库中未能充分比对的OTU,在NCBI GenBank数据库中进行手工比对。通过Canoco 4.5软件对土壤理化性质和真菌群落结构及多样性进行冗余分析(Redundancy analysis,RDA)。
1.5 数据处理
试验数据计算和图表绘制在Microsoft Excel 2007和SigmaPlot 12.0软件上进行,使用SPSS 21.0软件进行处理间差异的显著性检验(One-way,P< 0.05)。图表中试验结果采用平均值±标准差表示(n = 3)。不同数据间的相关性分析采用常见的一元回归模型进行。真菌丰度采用常用对数转换来表示。本研究所获得原始测序数据已上传至NCBI SRA数据库,登录号为SRP145523。
2 结果与分析
2.1 设施黄瓜连作对土壤真菌丰度和真细比的影响
温室黄瓜连作显著改变了土壤真菌数量(图1)。较CC1相比,CC10和CC15真菌数量分别显著增加4.42%和4.18%,但CC5和CC20处理均无显著差异。各处理土壤真细比变化趋势与真菌数量一致,CC10和CC15处理较CC1分别显著增加3.45%和3.77%。同时,回归分析证明土壤真菌数量和真细比均表现出随连作年限延长先增加后降低的抛物线趋势(P< 0.05)。
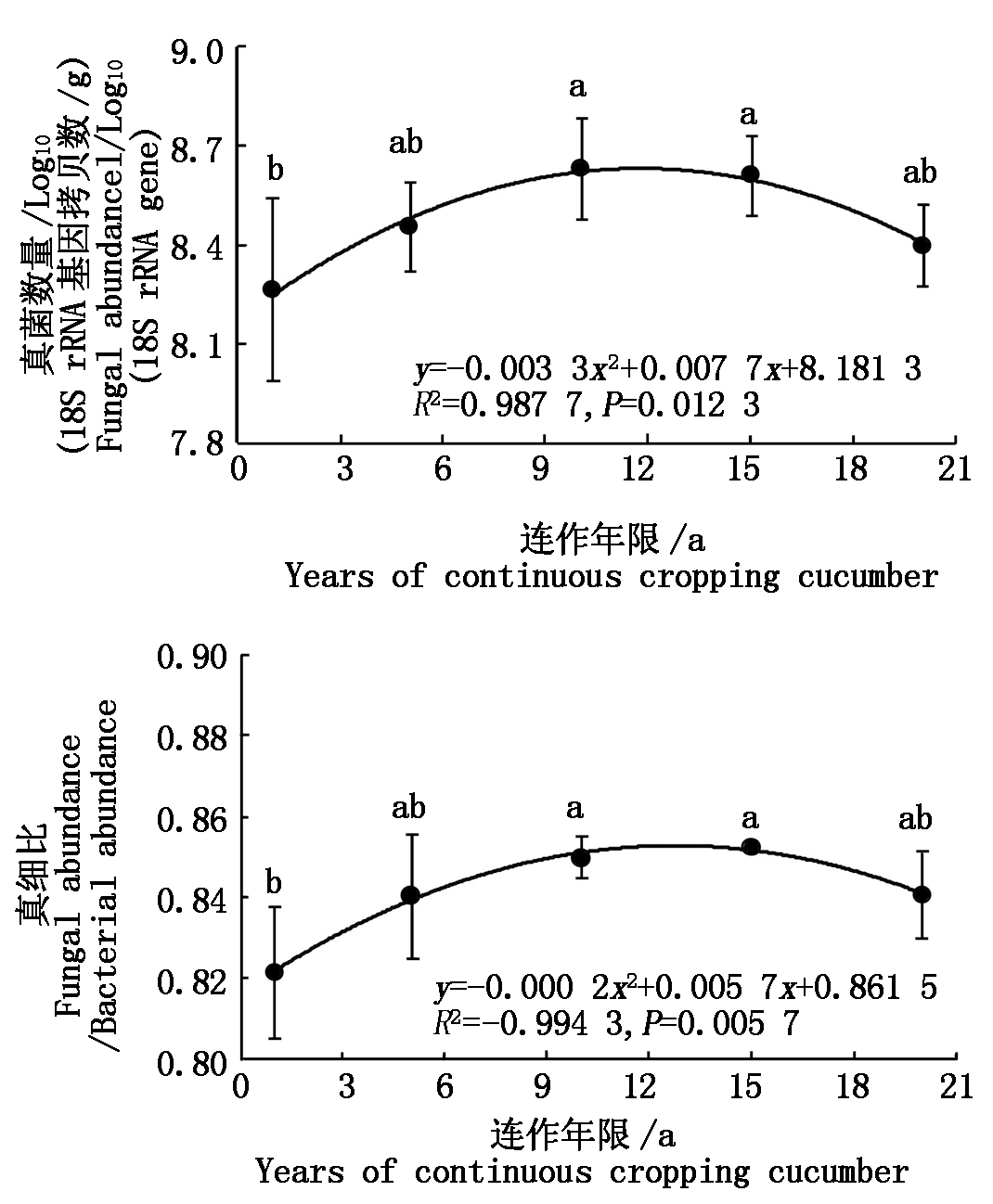
不同小写字母表示差异显著(P<0.05)。表1-4同。
Different small letters mean significant difference at 0.05 level.The same as Tab.1-4.
图1 温室黄瓜连作对土壤真菌数量和真细比的影响
Fig.1 Effects of continuous cropping cucumber on soil fungal abundance and the ratio of fungi to bacteria
2.2 设施黄瓜连作对土壤真菌群落α多样性和β多样性的影响
由表1可知,CC5~CC20处理土壤真菌群落OTU数量和群落的Shannon、Simpson、Ace和Chao1指数总体上与CC1均无显著差异,表明真菌群落的α多样性并不受温室黄瓜长期连作影响。但从群落OTU组成上来看(图2),CC1~CC20各处理真菌群落共享的OTU数量为118个,独有的OTU数量,5个处理依次为8,3,1,2,2个,分别占总观测到OTU数量的5.52%,2.12%,0.73%,1.48%和1.31%,随着温室黄瓜连作年限延长,群落中独有的OTU比例降低,显示温室黄瓜连作改变了真菌群落组成。层级聚类(图3-A)和PCoA分析(图3-B)证明,温室黄瓜连作显著改变了真菌群落的β多样性,且各连作年限处理间真菌群落结构差异达到显著水平。
表1 温室黄瓜连作对土壤真菌群落α多样性的影响
Tab.1 Effects of continuous cropping cucumber on OTU-based α diversity of soil fungal community
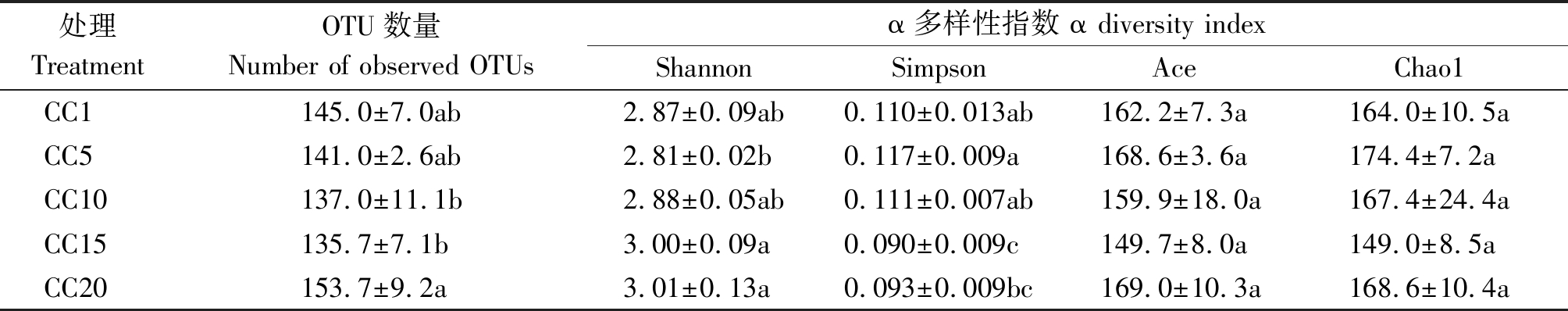
处理TreatmentOTU数量Number of observed OTUsα多样性指数 α diversity indexShannonSimpsonAceChao1CC1145.0±7.0ab2.87±0.09ab0.110±0.013ab162.2±7.3a164.0±10.5aCC5141.0±2.6ab2.81±0.02b0.117±0.009a168.6±3.6a174.4±7.2aCC10137.0±11.1b2.88±0.05ab0.111±0.007ab159.9±18.0a167.4±24.4aCC15135.7±7.1b3.00±0.09a0.090±0.009c149.7±8.0a149.0±8.5aCC20153.7±9.2a3.01±0.13a0.093±0.009bc169.0±10.3a168.6±10.4a
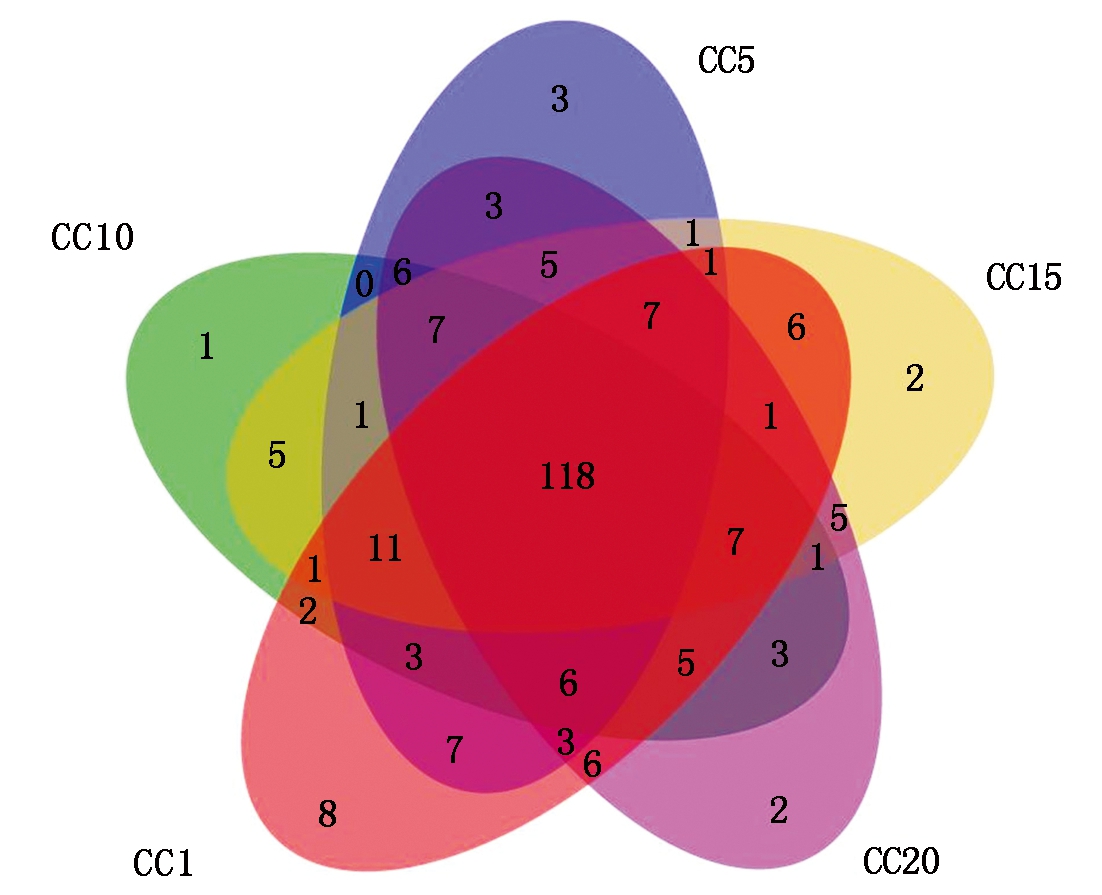
图2 不同温室黄瓜连作年限下土壤真菌群落共有和独有的OTU数目
Fig.2 Numbers of shared and unique OTUs in soil fungal communities in the condition of various years of continuous cropping cucumber
2.3 设施黄瓜连作对土壤真菌群落成员的影响
真菌群落中平均相对丰度超过1%的真菌门分别为子囊菌门、未分类真菌(Unclassified_k_Fungi)、未分类真菌(Norank_k_Fungi)、担子菌门、纤毛门和未分类真核生物(Unclassified_d_Eukaryota),这6个门共占总回收序列量的98.48%(表2)。其中,子囊菌门是所有供试土壤中最具优势的真菌门,其平均相对丰度占到总回收序列的87.22%,但各连作年限处理下子囊菌门的平均相对丰度并无显著差异。与CC1相比,CC5~CC20处理担子菌门平均相对丰度无显著变化。CC20处理纤毛门平均相对丰度较CC1显著增加302.20%,但CC5~CC15无显著差异。
在目水平下,群落中平均相对丰度超过0.01%的真菌目共有18个,占总回收序列量的98.20%(表3);平均相对丰度超过10%的真菌目共有4个,分别为小囊菌目、盘菌目、未分类子囊菌(Norank_p_Ascomycota)和粪壳菌目,占总回收序列量的70.05%。小囊菌目平均相对丰度在CC5~CC20下较CC1分别显著增加159.35%,148.99%,85.20%和60.20%,且整体上随连作年限延长先增加后降低。温室黄瓜连作显著降低了未分类子囊菌平均相对丰度,CC5~CC20与CC1相比降幅分别达到54.62%,40.45%,55.46%和40.37%。粪壳菌目平均相对丰度表现出随连作年限延长先降低后增加的趋势,CC5、CC10和CC15处理与CC1相比分别显著降低59.05%,55.06%和30.27%,而CC20处理则显著增加34.54%。与此同时,爪甲团囊菌目、肉座菌目和Conthreep的平均相对丰度也均随着连作年限延长呈现显著变化。
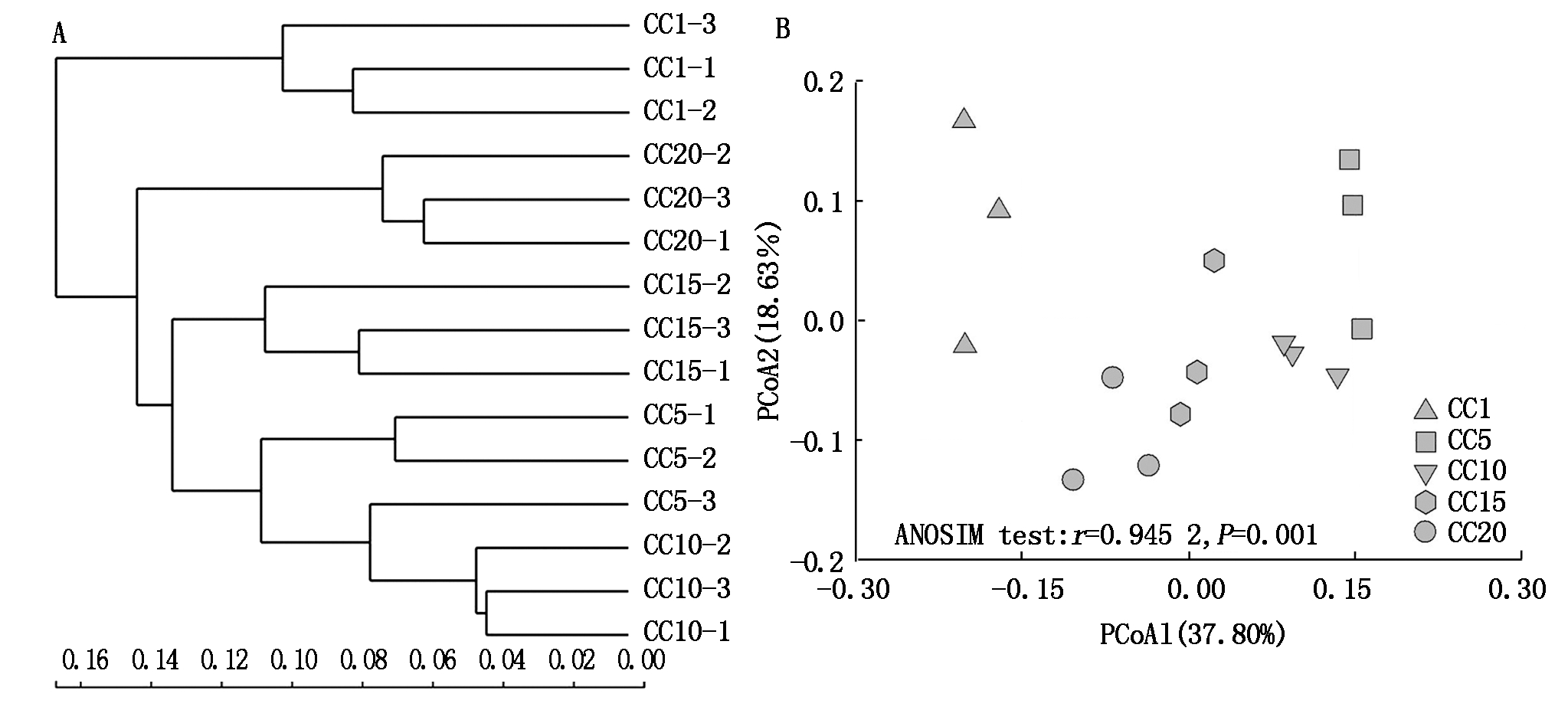
图3 土壤真菌群落在OTU水平的层级聚类(A)和主成分分析(B)
Fig.3 OTU-based hierarchical clustering(A)and PCoA analysis(B)of soil fungal community
表2 温室黄瓜连作对门水平下土壤真菌群落成员平均相对丰度的影响
Tab.2 Effects of continuous cropping cucumber on average relative abundances of fungal community members at phylum level %
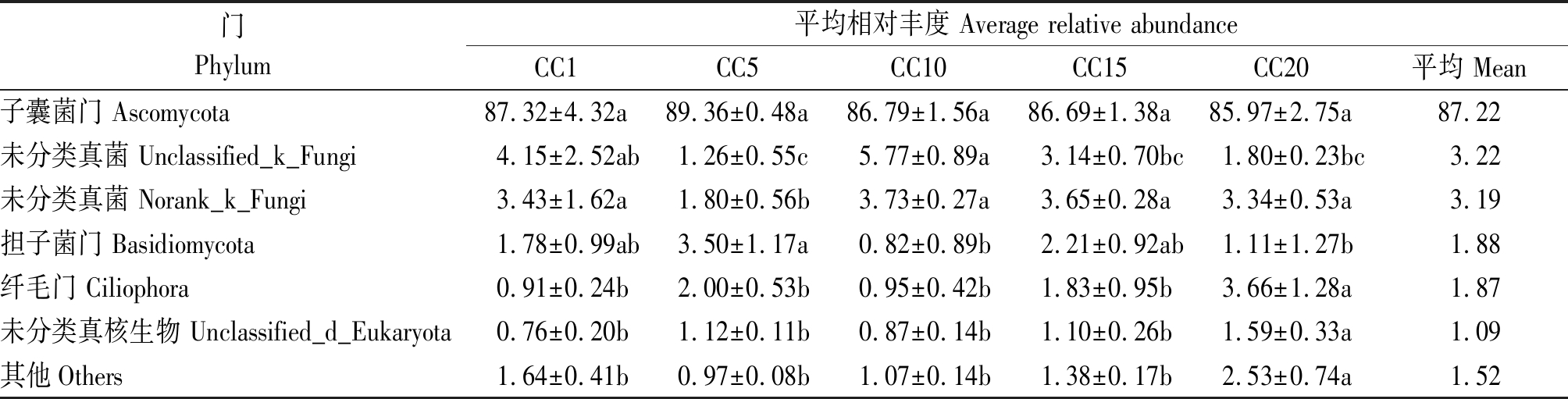
门Phylum平均相对丰度 Average relative abundanceCC1CC5CC10CC15CC20平均 Mean子囊菌门 Ascomycota87.32±4.32a89.36±0.48a86.79±1.56a86.69±1.38a85.97±2.75a87.22未分类真菌 Unclassified_k_Fungi4.15±2.52ab1.26±0.55c5.77±0.89a3.14±0.70bc1.80±0.23bc3.22未分类真菌 Norank_k_Fungi3.43±1.62a1.80±0.56b3.73±0.27a3.65±0.28a3.34±0.53a3.19担子菌门 Basidiomycota1.78±0.99ab3.50±1.17a0.82±0.89b2.21±0.92ab1.11±1.27b1.88纤毛门 Ciliophora0.91±0.24b2.00±0.53b0.95±0.42b1.83±0.95b3.66±1.28a1.87未分类真核生物 Unclassified_d_Eukaryota0.76±0.20b1.12±0.11b0.87±0.14b1.10±0.26b1.59±0.33a1.09其他Others1.64±0.41b0.97±0.08b1.07±0.14b1.38±0.17b2.53±0.74a1.52
表3 温室黄瓜连作对目水平下土壤真菌群落成员平均相对丰度的影响
Tab.3 Effects of continuous cropping cucumber on average relative abundances of fungal community members at order level %
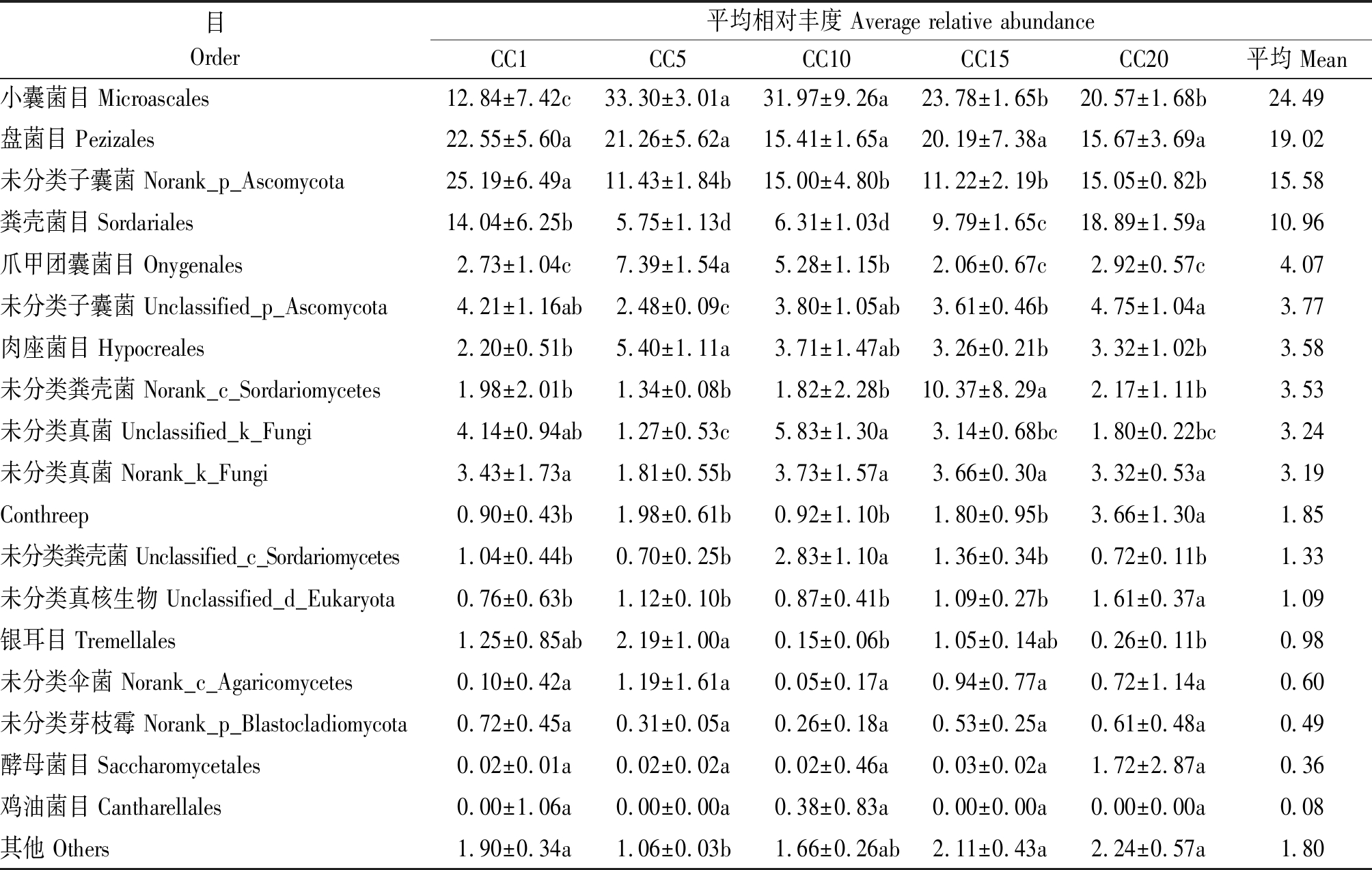
目Order平均相对丰度 Average relative abundanceCC1CC5CC10CC15CC20平均 Mean小囊菌目 Microascales12.84±7.42c33.30±3.01a31.97±9.26a23.78±1.65b20.57±1.68b24.49盘菌目 Pezizales22.55±5.60a21.26±5.62a15.41±1.65a20.19±7.38a15.67±3.69a19.02未分类子囊菌 Norank_p_Ascomycota25.19±6.49a11.43±1.84b15.00±4.80b11.22±2.19b15.05±0.82b15.58粪壳菌目 Sordariales14.04±6.25b5.75±1.13d6.31±1.03d9.79±1.65c18.89±1.59a10.96爪甲团囊菌目 Onygenales2.73±1.04c7.39±1.54a5.28±1.15b2.06±0.67c2.92±0.57c4.07未分类子囊菌 Unclassified_p_Ascomycota4.21±1.16ab2.48±0.09c3.80±1.05ab3.61±0.46b4.75±1.04a3.77肉座菌目 Hypocreales2.20±0.51b5.40±1.11a3.71±1.47ab3.26±0.21b3.32±1.02b3.58未分类粪壳菌 Norank_c_Sordariomycetes1.98±2.01b1.34±0.08b1.82±2.28b10.37±8.29a2.17±1.11b3.53未分类真菌 Unclassified_k_Fungi4.14±0.94ab1.27±0.53c5.83±1.30a3.14±0.68bc1.80±0.22bc3.24未分类真菌 Norank_k_Fungi3.43±1.73a1.81±0.55b3.73±1.57a3.66±0.30a3.32±0.53a3.19Conthreep0.90±0.43b1.98±0.61b0.92±1.10b1.80±0.95b3.66±1.30a1.85未分类粪壳菌 Unclassified_c_Sordariomycetes1.04±0.44b0.70±0.25b2.83±1.10a1.36±0.34b0.72±0.11b1.33未分类真核生物 Unclassified_d_Eukaryota0.76±0.63b1.12±0.10b0.87±0.41b1.09±0.27b1.61±0.37a1.09银耳目 Tremellales1.25±0.85ab2.19±1.00a0.15±0.06b1.05±0.14ab0.26±0.11b0.98未分类伞菌 Norank_c_Agaricomycetes0.10±0.42a1.19±1.61a0.05±0.17a0.94±0.77a0.72±1.14a0.60未分类芽枝霉 Norank_p_Blastocladiomycota0.72±0.45a0.31±0.05a0.26±0.18a0.53±0.25a0.61±0.48a0.49酵母菌目 Saccharomycetales0.02±0.01a0.02±0.02a0.02±0.46a0.03±0.02a1.72±2.87a0.36鸡油菌目 Cantharellales0.00±1.06a0.00±0.00a0.38±0.83a0.00±0.00a0.00±0.00a0.08其他 Others1.90±0.34a1.06±0.03b1.66±0.26ab2.11±0.43a2.24±0.57a1.80
在属水平上,群落中最丰富的前20个真菌属共占总回收序列量的96%左右(表4)。平均相对丰度超过10%的真菌属有4个,分别为假埃希氏菌属、Lasiobolidium、赭霉属和毛壳菌属,这4个属共占总回收序列量的64.24%。假埃希氏菌属平均相对丰度在CC1处理下最低,CC5~CC20处理较其分别显著增加177.26%,159.57%,97.65%和65.16%,并在整体上表现出随连作年限延长先增加后降低的趋势。Lasiobolidium平均相对丰度在各连作年限处理下无显著变化。与CC1相比,温室黄瓜连作年限增加显著降低了赭霉属的平均相对丰度。毛壳菌属的平均相对丰度随连作年限延长先降低后增加,CC5、CC10和CC15处理与CC1相比分别显著降低58.60%,56.25%和30.88%,而CC20显著增加35.51%。CC5处理与CC1相比显著降低了Gelatinomyce、Polychytrium和下梳霉属的平均相对丰度,但显著增加了帚枝霉属和隐囊菌属的平均相对丰度。CC15处理下小不整球壳属平均相对丰度显著高于其他处理。CC20处理下根生壶菌属平均相对丰度显著高于其他处理。CC10处理较CC1显著增加了Arachniotus、粪盘菌属、瓶毛壳属和Rhodoveronaea的平均相对丰度。此外, 温室生产中常见的2种土传致病真菌丝核菌属和镰刀菌属也被检出, 但二者的平均相对丰度均较低(<1%)。不同连作年限处理下丝核菌属平均相对丰度并无显著差异。镰刀菌属平均相对丰度在CC15处理下最高, 但与CC1相比无显著变化。
2.4 土壤理化性质与真菌群落和群落成员的关系
丰度最高的前20个真菌属中有12个与1个或多个土壤理化因子呈显著或极显著的线性相关关系(表5)。假埃希氏菌属与有效磷和速效钾呈显著正相关; 赭霉属与有机质、有效磷和速效钾呈极显著负相关; 小不整球壳属与硝态氮呈极显著正相关; Polychytrium、下梳霉属和Rhodoveronaea与pH值呈显著或极显著负相关; Sarocladium和Arachniotus分别于速效钾和pH值呈显著正相关; Rhizophydium与有机质、有效磷和电导度呈显著或极显著正相关; 隐球菌属与全氮呈显著负相关;丝核菌属与速效钾呈显著正相关; Remersonia与pH值呈显著负相关。RDA分析表明(图4),土壤硝态氮(R2 =0.721 2,P=0.001)、速效钾(R2 =0.699 8,P=0.002)和有机质(R2 =0.391 0,P=0.048)含量显著驱动着温室黄瓜连作土壤的真菌群落结构变化,其余土壤理化因子(全氮、铵态氮、速效磷、pH值和电导度)对真菌群落结构变化的影响并不显著(P>0.05)。
表4 温室黄瓜连作对最丰富的20个真菌属的平均相对丰度的影响
Tab.4 Effects of continuous cropping cucumber on average relative abundances of top 20 abundant genera in soil fungal community %

属Genus平均相对丰度 Average relative abundanceCC1CC5CC10CC15CC20平均 Mean假埃希氏菌属 Pseudallescheria11.08 ± 2.11d30.72 ± 2.32a28.76 ± 1.43a21.90 ± 1.56b18.30 ± 1.49c22.15Lasiobolidium19.80 ± 8.15a18.43 ± 6.37a11.30 ± 1.83a17.33 ± 7.52a12.84 ± 3.88a15.94赭霉属 Ochroconis25.18 ± 3.35a11.38 ± 1.85b15.02 ± 0.86b11.20 ± 2.24b14.99 ± 0.81b15.55毛壳菌属 Chaetomium13.60 ± 0.64b5.63 ± 1.14d5.95 ± 0.42d9.40 ± 1.60c18.43 ± 1.57a10.60Gelatinomyces4.21 ± 0.39ab2.42 ± 0.10c3.82 ± 0.25ab3.58 ± 0.47b4.75 ± 1.02a3.76小不整球壳属 Plectosphaerella1.98 ± 0.64b1.35 ± 0.12b1.83 ± 0.14b10.42 ± 8.26a2.15 ± 1.10b3.54Polychytrium4.15 ± 2.52ab1.26 ± 0.55c5.77 ± 0.89a3.14 ± 0.70bc1.80 ± 0.23bc3.22下梳霉属 Coemansia3.43 ± 1.62a1.80 ± 0.56b3.73 ± 0.27a3.65 ± 0.28a3.34 ± 0.53a3.19帚枝霉属 Sarocladium1.57 ± 0.38b4.95 ± 1.30a3.32 ± 1.63ab2.53 ± 0.25b3.10 ± 1.03ab3.09Arachniotus2.09 ± 1.06b5.74 ± 1.25a4.10 ± 1.00a1.60 ± 0.60b1.72 ± 0.51b3.05粪盘菌属 Ascobolus2.48 ± 0.45b2.65 ± 0.86ab3.83 ± 0.98a2.59 ± 0.50ab2.65 ± 0.28ab2.84瓶毛壳属 Lophotrichus1.84 ± 0.22b2.55 ± 0.88ab3.30 ± 0.24a1.88 ± 0.17b2.32 ± 0.28b2.38Rhodoveronaea1.03 ± 0.60b0.71 ± 0.25b2.84 ± 0.37a1.38 ± 0.34b0.73 ± 0.13b1.34根生壶菌属 Rhizophydium0.76 ± 0.20b1.12 ± 0.11b0.87 ± 0.14b1.10 ± 0.26b1.59 ± 0.33a1.09隐囊菌属 Aphanoascus0.62 ± 0.12c1.64 ± 0.23a1.17 ± 0.33b0.45 ± 0.10c1.19 ± 0.09b1.01隐球菌属 Cryptococcus1.24 ± 0.88ab2.22 ± 1.04a0.14 ± 0.04b1.02 ± 0.12b0.26 ± 0.09b0.98丝核菌属 Rhizoctonia0.10 ± 0.06a1.19 ± 1.60a0.04 ± 0.01a0.95 ± 0.79a0.73 ± 1.16a0.60Microallomyces0.73 ± 0.40a0.31 ± 0.05a0.27 ± 0.20a0.52 ± 0.24a0.62 ± 0.49a0.49镰刀菌属 Fusarium0.58 ± 0.15ab0.45 ± 0.31ab0.38 ± 0.15ab0.73 ± 0.23a0.21 ± 0.04b0.47Remersonia0.45 ± 0.17a0.13 ± 0.09b0.34 ± 0.03a0.44 ± 0.05a0.52 ± 0.03a0.38
表5 最丰富的20个真菌属的平均相对丰度与土壤理化性质的线性相关分析
Tab.5 Linear correlations between average relative abundances of top 20 abundant genera and soil physiochemical properties
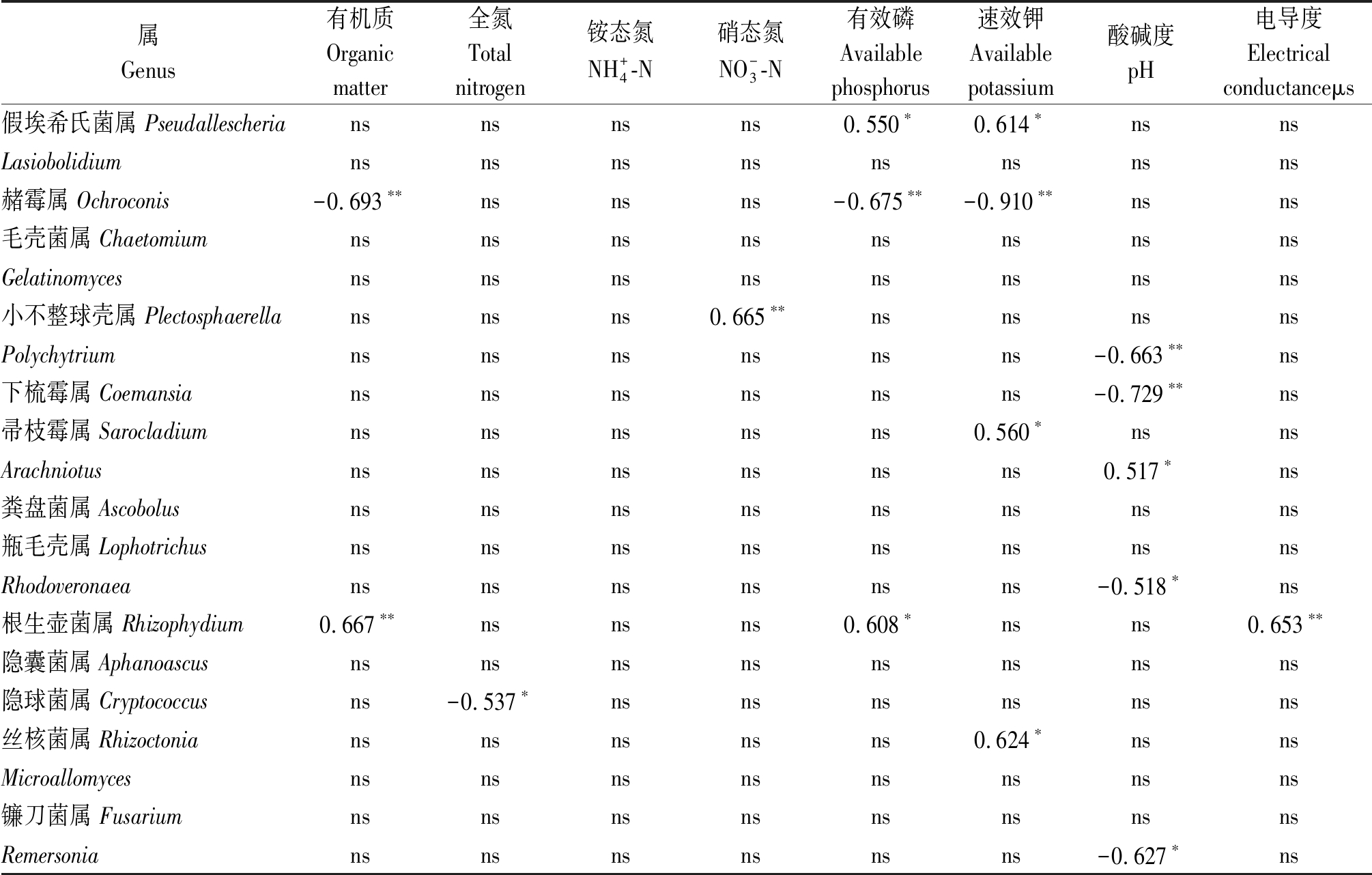
属Genus有机质Organicmatter全氮Totalnitrogen铵态氮NH+4-N硝态氮NO-3-N有效磷Availablephosphorus速效钾Availablepotassium酸碱度pH电导度Electricalconductanceμs假埃希氏菌属 Pseudallescheriansnsnsns0.550∗0.614∗nsnsLasiobolidiumnsnsnsnsnsnsnsns赭霉属 Ochroconis-0.693∗∗nsnsns-0.675∗∗-0.910∗∗nsns毛壳菌属 ChaetomiumnsnsnsnsnsnsnsnsGelatinomycesnsnsnsnsnsnsnsns小不整球壳属 Plectosphaerellansnsns0.665∗∗nsnsnsnsPolychytriumnsnsnsnsnsns-0.663∗∗ns下梳霉属 Coemansiansnsnsnsnsns-0.729∗∗ns帚枝霉属 Sarocladiumnsnsnsnsns0.560∗nsnsArachniotusnsnsnsnsnsns0.517∗ns粪盘菌属 Ascobolusnsnsnsnsnsnsnsns瓶毛壳属 LophotrichusnsnsnsnsnsnsnsnsRhodoveronaeansnsnsnsnsns-0.518∗ns根生壶菌属 Rhizophydium0.667∗∗nsnsns0.608∗nsns0.653∗∗隐囊菌属 Aphanoascusnsnsnsnsnsnsnsns隐球菌属 Cryptococcusns-0.537∗nsnsnsnsnsns丝核菌属 Rhizoctoniansnsnsnsns0.624∗nsnsMicroallomycesnsnsnsnsnsnsnsns镰刀菌属 FusariumnsnsnsnsnsnsnsnsRemersoniansnsnsnsnsns-0.627∗ns
注:ns.无显著相关性;*.P<0.05;**.P<0.01。
Note: ns.No significant;*.P<0.05; **. P<0.01.
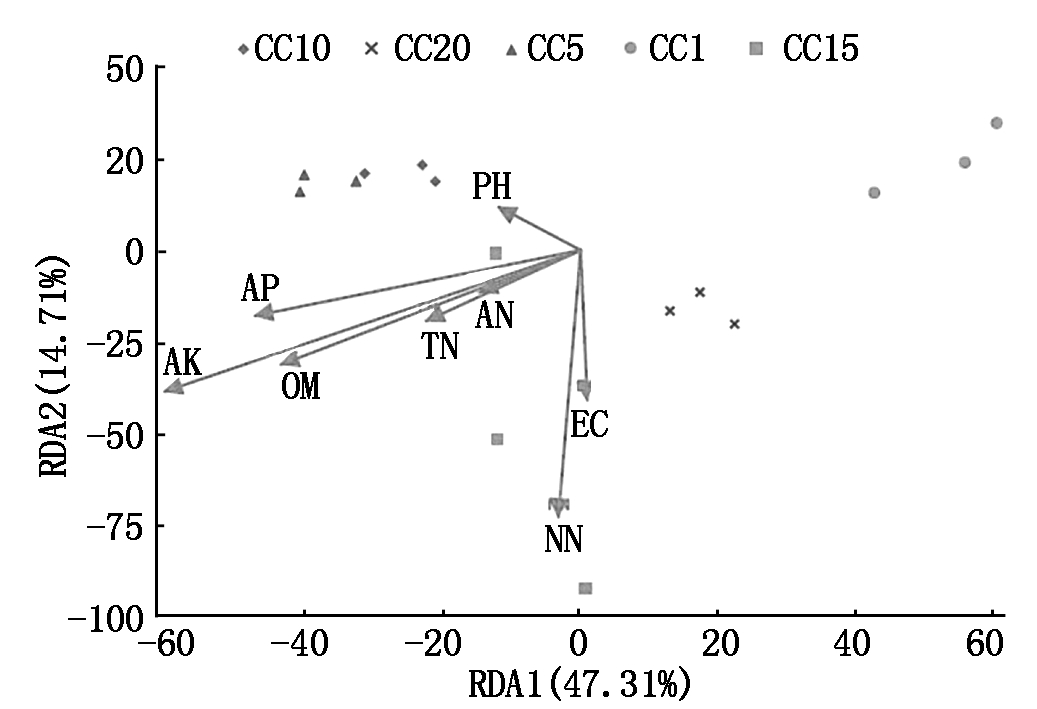
OM.有机质;TN.全氮;AN.铵态氮;NN.硝态氮;AP.有效磷;AK.有效钾;pH.酸碱度;EC.电导度。
OM,TN,AN,NN,AP,AK,pH and EC represent organic matter,total nitrogen,ammonium nitrogen,nitrate nitrogen,available phosphorus,available potassium,pH,and electrical conductance,respectively.
图4 土壤真菌群落结构与土壤理化因子的关系(冗余分析)
Fig.4 The relationship of soil fungal community structureand soil physiochemical factors based on RDA analysis
3 结论与讨论
长期集约化连作生产常常导致作物生长发育受阻,经济产量和品质降低,且土传病害滋生,其中真菌型土传病害表现尤为普遍[28-30]。大量基于可培养微生物和分子生物学方法进行的研究报道业已证明,土壤微生物区系与土传真菌型致病菌积累和病害发生息息相关[31-38]。因此,本研究将温室黄瓜连作条件下土壤真菌数量和群落结构作为研究的重点。实时荧光定量PCR结果表明,温室黄瓜连作显著影响了土壤真菌数量,并表现出随连作年限延长先增加后降低的趋势,这与Zhou等[14]的研究结果一致。真细比通常被认为是表征土壤肥力和健康的重要指标[39],与真菌数量变化类似,真细比也随连作年限延长先增后减,显示土壤微生物区系结构受温室黄瓜连作的显著影响。值得注意的是,尽管早前较多的研究也证实长期温室连作常常增加土壤真菌数量并提高真细比[40-41],但本研究结果暗示土壤真菌数量和微生物区系结构受连作年限长短调控。有研究发现,一些作物的产量常常在一定连作年限后逐步得到自我恢复,并且连作障碍程度趋于减轻甚至消失,如大豆[42-43]和棉花[44-45]等,但其中相关的潜在机制尚不清晰,猜测可能与作物和土壤微生物二者在长期连作种植过程中的相互适应有关[45]。
高通量测序显示,真菌群落的β多样性与α多样性相比对温室黄瓜连作更为敏感。不同连作年限下土壤样品真菌群落结构明显变化,在层级聚类分析和PCoA图中,CC5~CC20处理土壤样品与CC1区分明显,这些与早前学者在温室黄瓜连作研究中通过PCR-DGGE方法所取得的结果一致[13-14]。但Yao等[15]基于群落水平生理多样性(CLPP)和随机扩增多态性DNA(RAPD)的方法研究表明,长期温室黄瓜连作显著降低了土壤微生物多样性,推测供试土壤性质的不同以及方法学上的差异造成了这种不一致的研究结果。就真菌群落成员而言,少数的优势成员支配着整个真菌群落,假埃希氏菌属、Lasiobolidium、赭霉属和毛壳菌属共占总回收序列量的64.24%,它们作为真菌群落组成的基石在最大程度上决定着整个真菌群落对温室黄瓜连作的响应。假埃希氏菌属作为在属水平下最丰富的群落成员,其平均相对丰度随连作年限延长先增后降,这与土壤真菌数量变化一致。高通量测序结果也证明不同真菌群落成员对温室黄瓜连作响应的差异性,这可能与土壤理化性质变化有关,不同的微生物种属适应不同的生态位。相关分析的确证明,多数的优势真菌群落成员均与土壤理化因子之间存在着广泛的相关性。
微生物常常行使各种各样的土壤生态功能并能够提供生态服务。假埃希氏菌属是土壤N2O排放的重要贡献者[46],早前的研究也证明,自毒物质(如香草醛)的添加能够显著降低黄瓜幼苗根际真菌群落中假埃希氏菌属的平均相对丰度[47]。Lasiobolidium已被证明是设施番茄长期连作土壤中的优势真菌属[48],这与本研究结果类似。高通量测序结果显示,刨花楠(Machilus pauhoi)长期种植显著改变土壤优势真菌赭霉属平均相对丰度[49]。毛壳菌是一种典型生防菌,可以产生抗生素和细胞壁降解酶,对一些土传、气传病害起到防治作用[50]。在本试验中,CC5、CC10、CC15处理下毛壳菌平均相对丰度较CC1显著降低,而在CC20处理下又显著回升,表明土壤一些有益菌的丰度受连作年限影响,但连作20 a以后毛壳菌平均相对丰度出现显著回升的内在原因尚需进一步研究。此类现象在东北地区大豆中也有所报道,魏巍[43]研究表明,长期连作可形成根腐病抑制性土壤,减轻大豆根腐病发病程度,抑制病原镰孢菌的生长。另外,前人的研究结果表明,随着连作年限的增加,土壤中致病性真菌增多。马云华等[51]对温室黄瓜的研究发现,随着连作年限的增加,土壤中引起黄瓜枯萎病的尖孢镰刀菌逐渐成为优势真菌生理群。本研究的结果显示,土壤真菌群落中镰刀菌(Fusarium sp.)的平均相对丰度较低,且CC5~CC20处理与CC1相比并未出现显著差异;此外,另一种常见土传致病菌丝核菌(Rhizoctonia sp.)的平均相对丰度在不同连作年限下也无显著变化。推测可能与试验区内菜农长期且高量的农药使用有关。有意思的是,尽管常见的土传致病真菌的丰度并未发生明显的改变,但是对细菌的高通量测序结果显示,一些重要的生防菌芽孢杆菌(Bacillus sp.)和假单胞菌(Pseudomonas sp.)的丰度随连作年限延长显著降低。因此,需要进一步阐明特征微生物在温室蔬菜连作条件下的行为特征及其与土壤抑病性/致病性的关系。
本研究证明,硝态氮、速效钾和有机质含量是驱动温室黄瓜连作土壤真菌群落结构变化的主要因子。前期的研究结果已经证明[25],长期的温室黄瓜连作显著增加了土壤硝态氮、速效钾和有机质的含量。作为微生物的主要生境以及物质和能源的供应池,土壤环境变化直接驱动微生物组成和结构的变化,土壤微生物也对土壤理化因子扰动做出快速响应。总的来看,本研究结果也显示了土壤真菌群落结构对由长期温室黄瓜连作所导致的复杂的土壤理化环境变化的快速响应。
[1] 兰挚谦,郑文德,林薇,马嘉伟,张凯歌,张雪艳. 不同土壤改良剂对番茄生长和土壤肥力的影响[J]. 河南农业科学,2019,48(5): 91-98. doi: 10.15933/j.cnki.1004-3268.2019.05.014.
Lan Z Q,Zheng W D,Lin W,Ma J W,Zhang K G,Zhang X Y. Effect of different soil amendment on the tomato growth characteristic and soil fertility[J]. Journal of Henan Agricultural Sciences,2019,48(5): 91-98.
[2] 张帆,李姝,肖达,赵静,王然,郭晓军,王甦.中国设施蔬菜害虫天敌昆虫应用研究进展[J].中国农业科学,2015,48(17): 3463-3476. doi: 10.3864/j.issn.0578-1752.2015.17.013.
Zhang F,Li S,Xiao D,Zhao J,Wang R,Guo X J,Wang S. Progress in pest management by natural enemies in greenhouse vegetables in China[J]. Scientia Agricultura Sinica,2015,48(17): 3463-3476.
[3] 李天来,杨丽娟.作物连作障碍的克服-难解的问题[J].中国农业科学,2016,49(5): 916-918. doi: 10.3864/j.issn.0578-1752.2016.05.011.
Li T L,Yang L J. Overcoming continuous cropping obstacles-the different problem[J].Scientia Agricultura Sinica,2016,49(5): 916-918.
[4] 刘星,邱慧珍,张文明,张春红,朱静,马兴,程万莉.甘肃中部沿黄灌区马铃薯连作对土壤化学和生物学性质的影响[J].中国生态农业学报,2017,25(4): 581-593. doi: 10.13930/j.cnki.cjea.160848.
Liu X,Qiu H Z,Zhang W M,Zhang C H,Zhu J,Ma X,Cheng W L. Effect of continuous potato monoculture on soil chemical and biological properties in Yellow River Irrigation Area in central Gansu Province[J]. Chinese Journal of Eco-Agriculture,2017,25(4): 581-593.
[5] Huang L F,Song L X,Xia X J,Mao W H,Shi K,Zhou Y H,Yu J Q. Plant-soil feedbacks and soil sickness: from mechanisms to application in agriculture[J]. Journal of Chemical Ecology,2013,39(2): 232-242. doi: 10.1007/s10886-013-0244-9.
[6] Li X G,Ding C F,Hua K,Zhang T L,Zhang Y N,Zhao L,Yang Y R,Liu J G,Wang X X. Soil sickness of peanuts is attributable to modifications in soil microbes induced by peanut root exudates rather than to direct allelopathy[J]. Soil Biology and Biochemistry,2014,78(11): 149-159. doi: 10.1016/j.soilbio.2014.07.019.
[7] Chen T,Liu S,Wu L K,Lin W X,Sampietro D A. Soil sickness: current status and future perspectives[J]. Allelopathy Journal,2015,36(2): 167-196.
[8] Huang H C,Chou C H,Erickson R S. Soil sickness and its control[J]. Allelopathy Journal,2006,18(1): 1-21.
[9] Li W H,Liu Q Z,Chen P. Effect of long-term continuous cropping of strawberry on soil bacterial community structure and diversity[J]. Journal of Integrative Agriculture,2018,17(11): 2570-2582. doi: 10.1016/S2095-3119(18)61944-6.
[10] Xiao W L,Wang Z X,Wu F Z,Zhou X G. Effects of soil improvement technology on soil quality in solar greenhouse[J]. Environmental Science and Pollution Research,2018,25(24): 24093-24100. doi: 10.1007/s11356-018-2321-7.
[11] Zhou X G,Liu J,Wu F Z. Soil microbial communities in cucumber monoculture and rotation systems and their feedback effects on cucumber seedling growth[J]. Plant and Soil,2017,415(1-2): 507-520. doi: 10.1007/s11104-017-3181-5.
[12] Yao Z Y,Xing J J,Gu H P,Wang H Z,Wu J J,Xu J M,Brookes P C. Development of microbial community structure in vegetable-growing soils from open-field to plastic-greenhouse cultivation based on the PLFA analysis[J]. Journal of Soils and Sediments,2016,16(8): 2041-2049. doi: 10.1007/s11368-016-1397-2.
[13] Zhou X G,Gao D M,Liu J,Qiao P L,Zhou X L,Lu H B,Wu X,Liu D,Jin X,Wu F Z. Changes in rhizosphere soil microbial communities in a continuously monocropped cucumber(Cucumis sativus L.)system[J]. European Journal of Soil Biology,2014,60: 1-8. doi: 10.1016/j.ejsobi.2013.10.005.
[14] Zhou X G,Wu F Z. Dynamics of the diversity of fungal and Fusarium communities during continuous cropping of cucumber in the greenhouse[J]. FEMS Microbiology Ecology,2012,80(2): 469-478. doi: 10.1111/j.1574-6941.2012.01312.x.
[15] Yao H Y,Jiao X D,Wu F Z. Effects of continuous cucumber cropping and alternative rotations under protected cultivation on soil microbial community diversity[J]. Plant and Soil,2006,284(1-2): 195-203. doi: 10.1007/s11104-006-0023-2.
[16] Bonanomi G,Antignani V,Capodilupo M,Scala F. Identifying the characteristics of organic soil amendments that suppress soilborne plant diseases[J]. Soil Biology and Biochemistry,2010,42(2): 136-144. doi: 10.1016/j.soilbio.2009.10.012.
[17] Zhou D B,Jing T,Chen Y F,Wang F,Qi D F,Feng R J,Xie J H,Li H P. Deciphering microbial diversity associated with Fusarium wilt-diseased and disease-free banana rhizosphere soil[J]. BMC Microbiology,2019,19: 161. doi: 10.1186/s12866-019-1531-6.
[18] Xiong W,Li R,Ren Y,Liu C,Zhao Q Y,Wu H S,Jousset A,Shen Q R. Distinct roles for soil fungal and bacterial communities associated with the suppression of vanilla Fusarium wilt disease[J]. Soil Biology and Biochemistry,2017,107: 198-207. doi: 10.1016/j.soilbio.2017.01.010.
[19] Xiong W,Zhao Q Y,Zhao J,Xun W B,Li R,Zhang R F,Wu H S,Shen Q R. Different continuous cropping spans significantly affect microbial community membership and structure in a vanilla-grown soil as revealed by deep pyrosequencing[J]. Microbial Ecology,2015,70(1): 209-218. doi: 10.1007/s00248-014-0516-0.
[20] Li X G,Ding C F,Zhang T L,Wang X X. Fungal pathogen accumulation at the expense of plant-beneficial fungi as a consequence of consecutive peanut monoculturing[J]. Soil Biology and Biochemistry,2014,72: 11-18. doi: 10.1016/j.soilbio.2014.01.019.
[21] Li J G,Ren G D,Jia Z J,Dong Y H. Composition and activity of rhizosphere microbial communities associated with healthy and diseased greenhouse tomatoes[J]. Plant and Soil,2014,485(1-2): 337-347. doi: 10.1007/s11104-014-2097-6.
[22] Lu L H,Yin S X,Liu X,Zhang W M,Gu T Y,Shen Q R,Qiu H Z. Fungal networks in yield-invigorating and-debilitating soils induced by prolonged potato monoculture[J]. Soil Biology and Biochemistry,2013,65: 186-194. doi: 10.1016/j.soilbio.2013.05.025.
[23] Chen M N,Li X,Yang Q L,Chi X Y,Pan L J,Chen N,Yang Z,Wang T,Wang M,Yu S L. Soil eukaryotic microorganism succession as affected by continuous cropping of peanut-pathogenic and beneficial fungi were selected[J]. PLoS One,2012,7(7): e40659. doi: 10.1371/journal.pone.0040659.
[24] 张瑞福,沈其荣.抑病型土壤的微生物区系特征及调控[J].南京农业大学学报,2012,35(5): 125-132. doi: 10.7685/j.issn.1000-2030.2012.05.014.
Zhang R F,Shen Q R. Characterization of the microbial flora and management to induce the disease suppressive soil[J]. Journal of Nanjing Agricultural University,2012,35(5): 125-132.
[25] Liu X,Zhang Y,Ren X J,Chen B H,Shen C W,Wang F. Long-term greenhouse vegetable cultivation alters the community structures of soil ammonia oxidizers[J]. Journal of Soils and Sediments,2019,19(2): 883-902. doi: 10.1007/s11368-018-2089-x.
[26] Rousk J,Bååth E,Brookes P C,Lauber C L,Lozupone C,Caporaso J G,Knight R,Fierer N. Soil bacterial and fungal communities across a pH gradient in an arable soil[J]. The ISME Journal,2010,4: 1340-1351. doi: 10.1038/ismej.2010.58.
[27] 董艳辉,于宇凤,温鑫,王亦学,聂园军,侯丽媛,李亚莉,刘江,任元,王育川,曹秋芬,吴慎杰,王斌,秦永军. 基于高通量测序的藜麦连作根际土壤微生物多样性研究[J]. 华北农学报,2019,34(2): 205-211. doi: 10.7668/hbnxb.201751218.
Dong Y H,Yu Y F,Wen X,Wang Y X,Nie Y J,Hou L Y,Li Y L,Liu J,Ren Y,Wang Y C,Cao Q F,Wu S J,Wang B,Qin Y J. Studies on diversity of rhizosphere microorganism in quinoa continuous cropping soil by high throughput sequencing[J]. Acta Agriculturae Boreali-Sinica,2019,34(2): 205-211.
[28] 杨珍,戴传超,王兴祥,李孝刚.作物土传真菌病害发生的根际微生物机制研究进展[J].土壤学报,2019,56(1): 12-22. doi: 10.11766/trxb2018032600578.
Yang Z,Dai C C,Wang X X,Li X G. Advance in research on rhizosphere microbial mechanisms of crop soil-borne fungal diseases[J]. Acta Pedologica Sinica,2019,56(1): 12-22.
[29] Shipton P J. Monoculture and soilborne plant pathogens[J]. Annual Review of Phytopathology,1977,15: 387-407. doi: 10.1146/annurev.py.15.090177.002131.
[30] Larkin R P. Soil health paradigms and implications for disease management[J]. Annual Review of Phytopathology,2015,53: 199-221. doi: 10.1146/annurev-phyto-080614-120357.
[31] Doran J W,Zeiss M R. Soil health and sustainability: managing the biotic component of soil quality[J]. Applied Soil Ecology,2000,15(1): 3-11. doi: 10.1016/s0929-1393(00)00067-6.
[32] Raaijmakers J M,Paulitz T C,Steinberg C,Alabouvette C,Mo⊇nne-Loccoz Y. The rhizosphere: a playground and battlefield for soilborne pathogens and beneficial microorganisms[J]. Plant and Soil,2009,321(1-2): 341-361. doi: 10.1007/s11104-008-9568-6.
[33] Omirou M,Rousidou C,Bekris F,Papadopoulou K K,Menkissoglou-Spiroudi U,Ehaliotis C,Karpouzas D G. The impact of biofumigation and chemical fumigation methods on the structure and function of the soil microbial community[J]. Microbial Ecology,2011,61(1): 201-213. doi: 10.1007/s00248-010-9740-4.
[34] van der Heijden M G A,Bardgett R D,van Straalen N M. The unseen majority: soil microbes as drivers of plant diversity and productivity in terrestrial ecosystems[J]. Ecology Letters,2008,11(3): 296-310. doi: 10.1111/j.1461-0248.2007.01139.x.
[35] Kennedy A C,Smith K L. Soil microbial diversity and the sustainability of agricultural soils[J]. Plant and Soil,1995,170(1): 75-86. doi: 10.1007/BF02183056.
[36] Schnitzer S A,Klironomos J N,HillerisLambers J,Kinkel L L,Reich P B,Xiao K,Rillig M C,Sikes B A,Callaway R M,Mangan S A,van Nes E H,Scheffer M. Soil microbes drive the classic plant diversity-productivity pattern[J].Ecology,2011,92(2): 296-303. doi: 10.1890/10-0773.1.
[37] Chaparro J M,Sheflin A M,Manter D K,Vivanco J M. Manipulating the soil microbiome to increase soil health and plant fertility[J]. Biology and Fertility of Soils,2012,48(5): 489-499. doi: 10.1007/s00374-012-0691-4.
[38] Welbaum G E,Sturz A V,Dong Z M,Nowak J. Managing soil microorganisms to improve productivity of agro-ecosystems[J]. Critical Reviews in Plant Sciences,2004,23(2): 175-193. doi: 10.1080/07352680490433295.
[39] Qin S H,Yeboah S,Cao L,Zhang J L,Shi S L,Liu Y H. Breaking continuous potato cropping with legumes improves soil microbial communities,enzyme activities and tuber yield[J]. PLoS One,2017,12(5): e0175934. doi: 10.1371/journal.pone.0175934.
[40] Fu H D,Zhang G X,Zhang F,Sun Z P,Geng G M,Li T L. Effects of continuous tomato monoculture on soil microbial properties and enzyme activities in a solar greenhouse[J]. Sustainability,2017,9: 317. doi: 10.3390/su9020317.
[41] Kang Y L,Jing F,Sun W Q,Liu J G,Jiang G Y. Soil microbial communities changed with a continuously monocropped processing tomato system[J]. Acta Agriculturae Scandinavica,Section B-Soil and Plant Science,2017,68(2): 149-160. doi: 10.1080/09064710.2017.1370124.
[42] Li C,Li X M,Kong W D,Wu Y,Wang J G. Effect of monoculture soybean on soil microbial community in the Northeast China[J]. Plant and Soil,2010,330(1-2): 423-433. doi: 10.1007/s11104-009-0216-6.
[43] 魏巍.大豆长期连作土壤对根腐病病原微生物的抑制作用[D].长春:中国科学院研究生院,2012.
Wei W. The suppressiveness caused by long-term continuous cropping of soybean on the root rot and pathogens[D]. Changchun: Graduate University of Chinese Academy of Sciences,2012.
[44] Gong L,He G X,Liu W G. Long-term cropping effects on agricultural sustainability in Alar Oasis of Xinjiang,China[J]. Sustainability,2016,8(1): 61. doi: 10.3390/su8010061.
[45] Zhang W,Long X Q,Huo X D,Chen Y F,Lou K. 16S rRNA-based PCR-DGGE analysis of actinomycete communities in fields with continuous cotton cropping in Xinjiang,China[J]. Microbial Ecology,2013,66(2): 385-393. doi: 10.1007/s00248-012-0160-5.
[46] Jirout J, imek M,Elhottov
imek M,Elhottov D. Fungal contribution to nitrous oxide emissions from cattle impacted soils[J]. Chemosphere,2013,90(2): 565-572. doi: 10.1016/j.chemosphere.2012.08.031.
D. Fungal contribution to nitrous oxide emissions from cattle impacted soils[J]. Chemosphere,2013,90(2): 565-572. doi: 10.1016/j.chemosphere.2012.08.031.
[47] Zhang J H,Yu H J,Ge X,Pan D D,Shen Y H,Qiao P L,Wu F Z,Zhou X G. Effects of vanillin on cucumber(Cucumis sativus L.)seedling rhizosphere fungal community composition[J]. Allelopathy Journal,2018,44(2): 169-179. doi: 10.26651/allelo.j/2018-44-2-1162.
[48] 马宁宁,李天来.设施番茄长期连作土壤微生物群落结构及多样性分析[J].园艺学报,2013,40(2): 255-264. doi: 10.16420/j.issn.0513-353x.2013.02.022.
Ma N N,Li T L. Effect of long-term continuous cropping of protected tomato on soil microbial community structure and diversity[J]. Acta Horticulturae Sinica,2013,40(2): 255-264.
[49] Guo X F. Diversity and community structure of fungi in the roots of Machilus pauhio in different age groups[J]. Applied Ecology and Environmental Research,2019,17(2): 2073-2083. doi: 10.15666/aeer/1702_20732083.
[50] 孟品品,刘星,邱慧珍,张文明,张春红,王蒂,张俊莲,沈其荣.连作马铃薯根际土壤真菌种群结构及其生物效应[J].应用生态学报,2012,23(11): 3079-3086. doi: 10.13287/j.1001-9332.2012.0464.
Meng P P,Liu X,Qiu H Z,Zhang W M,Zhang C H,Wang D,Zhang J L,Shen Q R. Fungal population structure and its biological effect in rhizosphere soil of continuously cropped potato[J]. Chinese Journal of Applied Ecology,2012,23(11): 3079-3086.
[51] 马云华,魏珉,王秀峰.日光温室连作黄瓜根区微生物区系及酶活性的变化[J].应用生态学报,2004,15(6): 1005-1008. doi: 10.13287/j.1001-9332.2004.0214.
Ma Y H,Wei M,Wang X F. Variation of microflora and enzyme activity in continuous cropping cucumber soil in solar greenhouse[J].Chinese Journal of Applied Ecology,2004,15(6): 1005-1008.