春季冻害是我国黄淮冬麦区和长江中下游冬麦区小麦生产的主要气象灾害之一。近年来,黄淮麦区和长江中下游冬麦区多次发生倒春寒,造成河南省中东部、江苏省北部、安徽省北部和陕西省关中等地部分品种出现穗部变形、缺粒、顶部不育等现象,导致小麦大面积减产。黄淮麦区近年来种植品种类型发生变化,从冬性品种逐渐往晚熟高产的偏春性品种转移,种植结构的改变加大了小麦遭受春季低温冻害的风险[1-2]。
研究表明,小麦抗寒性与植物细胞内渗透调节物质和保护酶系统关系密切。低温胁迫可使生物体的正常代谢遭到破坏,相关功能发生紊乱,可溶性糖和可溶性蛋白质大量积累,导致细胞液浓度提高,细胞冰点降低,从而增强机体的耐寒性[3-4]。同时,低温胁迫下植物的正常生理代谢失去平衡,体内积累大量的活性氧自由基,而逆境下活性氧清除系统又表现出相对较弱的清除能力,最终导致植株受害。植物体在长期的进化中形成了比较系统完善的活性氧清除酶系统(SOD、POD、CAT等),能防止植物体质膜受到伤害,研究表明,抗氧化酶活性的变化可作为植物抗寒性鉴定的生理指标[5]。
小麦的耐寒机制是一个复杂的生理过程,前人对苗期和越冬期小麦抗寒性研究较多,且大多侧重于一个生长发育时期,对小麦春季低温胁迫的研究也仅仅集中在低温胁迫下叶片抗寒相关的生理生化方面,鲜有结合幼穗分化阶段对春季低温胁迫下小麦幼穗抗寒生理机理的研究。前期研究发现,春季低温胁迫主要影响小麦的幼穗发育和结实性。因此,本试验以春季低温敏感和不敏感的2个小麦品种为材料,采用盆栽和人工模拟春季低温(倒春寒)胁迫的方法,研究小麦雌雄蕊原基分化期、药隔分化期和四分体形成期低温胁迫下小麦的结实特性与幼穗生理指标变化,分析抗倒春寒能力不同的小麦品种对低温胁迫的生理响应机制,为小麦抗倒春寒研究和品种改良提供理论参考。
1 材料和方法
1.1 试验材料
供试材料为前期筛选出的抗倒春寒小麦品种矮抗58和不抗倒春寒小麦品种郑麦366,均由河南省杂交小麦工程中心提供。
1.2 试验设计
试验于2017-2018年在河南省新乡市河南科技学院试验田进行。采用盆栽方法种植,盆栽土取大田耕层0~25 cm深处土壤,土壤平均碱解氮含量62.1 mg/kg ,速效磷含量11.1 mg/kg,速效钾含量132.5 mg/kg,有机质含量26.5 g/kg。盆直径22 cm,高25 cm,每盆装土8 kg,在盆底部钻3个小孔,保证盆内土壤与大田土壤的温度一致,水分和空气流通。将盆埋入大田后,盆内土壤与盆外大田齐平。土壤用水浇透沉实后于10月10日播种。2个品种分别设对照和低温处理2组,每组45盆。另外各播种10盆用于取样镜检跟踪幼穗发育进程,按照崔金梅等[6]的方法进行。
1.3 低温处理方法
从2018年3月1日开始,每隔2 d选取主茎和分蘖,调查矮抗58、郑麦366幼穗发育进程,2个小麦品种发育到颖片原基分化期后,将材料移至人工气候室中生长(气候室设置温度为白天22 ℃、夜晚13 ℃)。待小麦生长发育至雌雄蕊原基分化期、药隔分化期、四分体形成期后,分别将15盆材料移入人工气候箱中(人工气候箱设置:白天温度0 ℃,湿度为70%,时间12 h,光照强度30 000 lx;夜晚温度0℃,湿度70%,时间12 h,光照强度0 lx)处理24,48,72 h。以人工气候室中不处理盆栽为对照。处理结束后,将处理的材料分为两部分,一部分移至人工气候室继续生长,用于调查结实情况;另一部分取幼穗用于生理指标测定,将幼穗样品在冰盒上剪成小段,按0.5 g分装于冻存管中,迅速用液氮冷冻,于-80 ℃冰箱贮存备用。
1.4 抗氧化酶活性及可溶性蛋白质、可溶性糖、MDA含量的测定
采用蒽酮比色法测定可溶性糖含量,考马斯亮蓝G-250染色法测定可溶性蛋白质含量,愈创木酚法测定过氧化物酶(POD)活性,氮蓝四唑(NBT)光化还原法测定超氧化物歧化酶(SOD)活性,过氧化氢还原法测定过氧化氢酶(CAT)活性,硫代巴比妥酸显色法测定丙二醛(MDA)含量。生理指标的测定均采用苏州科铭生物科技有限公司试剂盒进行。
1.5 穗粒数测定
待处理过后移至人工气候室的对照和处理小麦生长至成熟期,调查单穗结实粒数,每处理随机调查50穗的结实粒数,求平均单穗穗粒数。
1.6 数据处理
采用Excel 2016进行数据整理及作图,使用SAS V8分析软件进行数据统计处理。
2 结果与分析
2.1 低温胁迫对参试材料幼穗结实性的影响
矮抗58和郑麦366幼穗发育进程鉴定结果见图1,其中雌雄蕊原基分化期低温胁迫处理72 h 2个品种的结实情况见图2。由表1 可知,2个小麦品种在低温胁迫后,随处理时间的延长,穗粒数均降低,且差异达到显著水平,但不同处理时间、不同品种间穗粒数降低幅度不同。其中,矮抗58在雌雄蕊原基分化期低温胁迫处理24,48,72 h后,穗粒数分别下降了6.52%,14.07%,22.37%,郑麦366则分别下降了37.14%,44.43%,64.71%;药隔分化期低温处理后,矮抗58穗粒数分别下降了3.74%,9.05%,13.71%,郑麦366分别下降了27.54%,37.80%,48.55%;四分体形成期低温处理后,矮抗58穗粒数分别下降了2.70%,4.70%,4.73%,郑麦366分别下降了12.39%,29.67%,32.30%。
随幼穗发育时期的延迟,同一低温处理时间下,2个参试材料穗粒数的降幅均表现下降趋势。说明雌雄蕊原基分化期对低温较敏感,此时期低温对穗粒数的影响更严重。同时,在3个低温处理时间、3个发育阶段,矮抗58的穗粒数均高于郑麦366,说明低温胁迫对郑麦366的影响大于矮抗58。
2.2 低温胁迫对参试材料幼穗可溶性蛋白质含量的影响
由图3可知, 低温处理后,2个小麦品种幼穗的可溶性蛋白质含量在雌雄蕊原基分化期、药隔分化期和四分体形成期均显著高于对照。雌雄蕊原基分化期和药隔分化期均呈缓慢上升-迅速上升-缓慢下降的趋势,在四分体形成期可溶性蛋白质含量呈现连续上升的趋势。
雌雄蕊原基分化期,矮抗58和郑麦366可溶性蛋白质含量在低温胁迫的24 h达到最大值,矮抗58较对照增加了116.67%,郑麦366较对照增加了103.78%;2个小麦品种幼穗在低温处理的24~72 h,可溶性蛋白质含量稍有下降,矮抗58下降幅度小于郑麦366(图3-A)。药隔分化期,低温处理下矮抗58与郑麦366幼穗中可溶性蛋白质含量出现最大增幅的时间不同,二者分别在低温处理的24,48 h达到最大值(图3-B)。四分体形成期,2个小麦品种可溶性蛋白质含量在0~24 h呈现连续快速上升的趋势,24 h后上升趋于平缓(图3-C)。
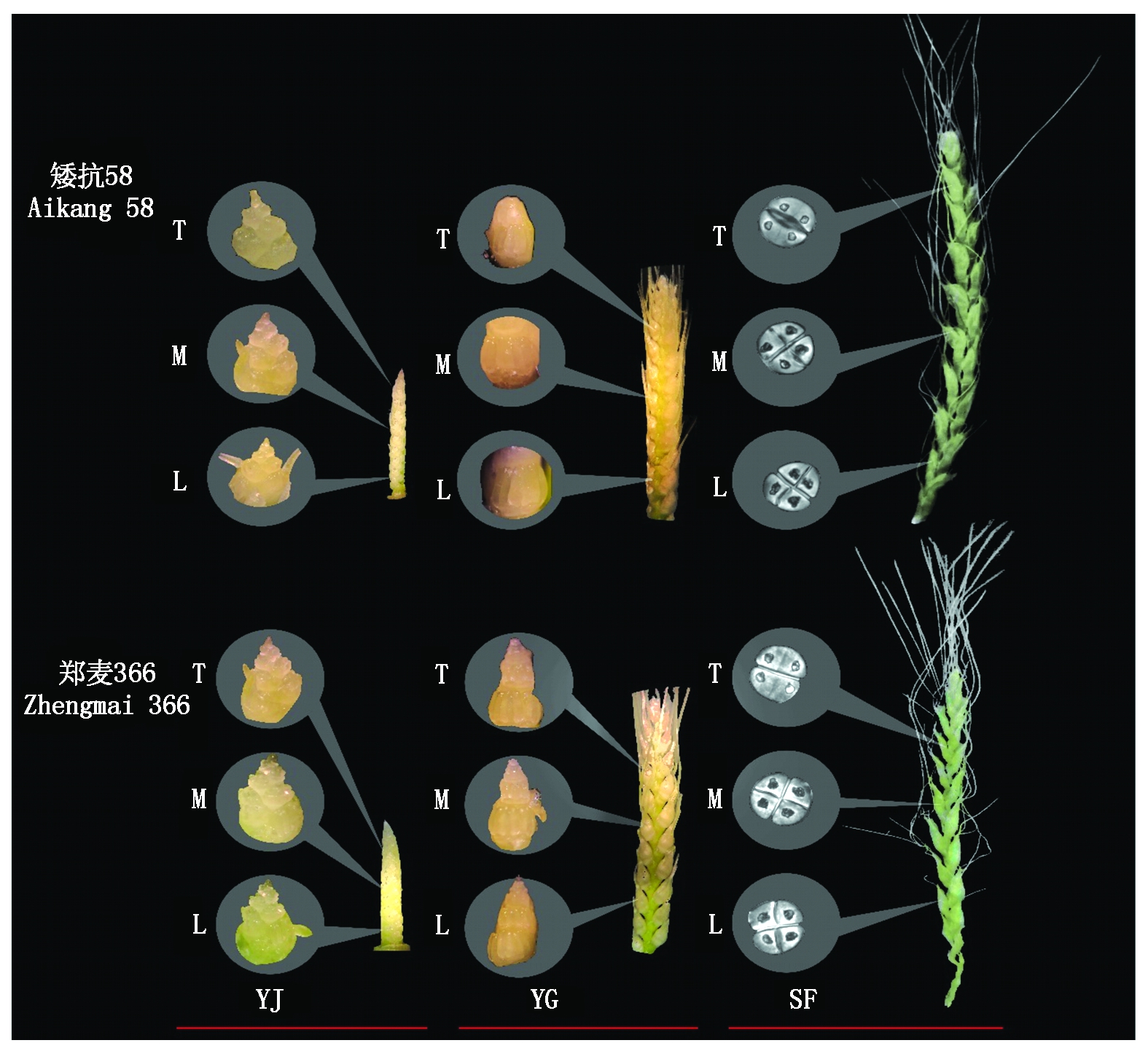
T.上部发育小花;M.中部发育小花;L.下部发育小花;YJ.雌雄蕊原基分化期;YG.药隔分化期;SF.四分体形成期。
T.Upper floret; M.The central flower; L.Lower floret;YJ.Stamens and pistils differentiation stage; YG.Anther separation stage; SF.Tetrad stage.
图1 不同小麦品种幼穗发育进程鉴定
Fig.1 Identification of development process of young ear of different wheat varieties
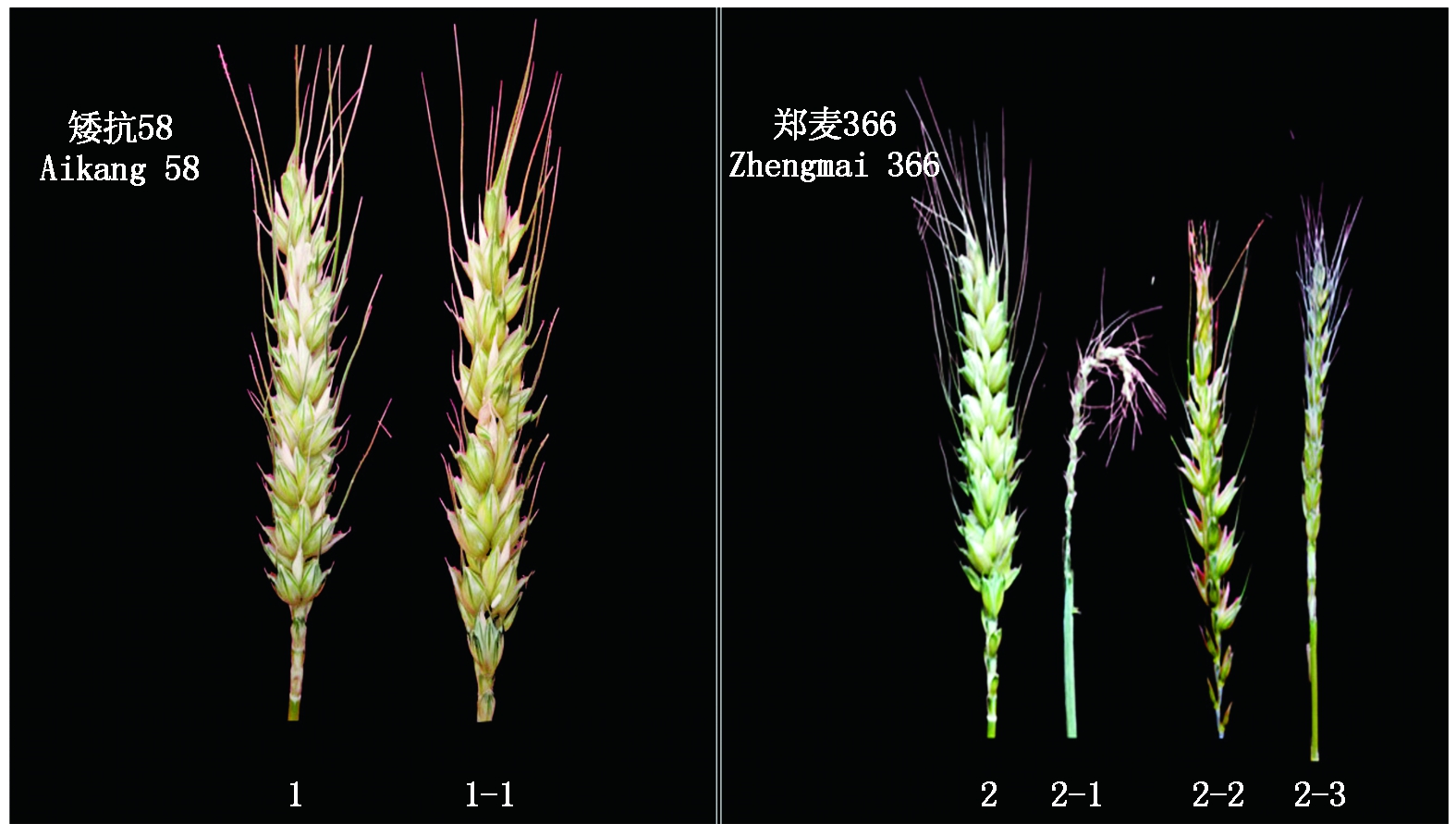
1.矮抗58对照;1-1.矮抗58低温处理;2.郑麦366对照;2-1,2-2,2-3.郑麦366低温处理。
1.Aikang 58 control; 1-1.Aikang 58 low temperature treatment;2.Zhengmai 366 control;2-1,2-2,2-3.Zhengmai 366 low temperature treatment.
图2 雌雄蕊原基分化期低温处理72 h矮抗58与郑麦366结实情况
Fig.2 The seed setting of Aikang 58 and Zhengmai 366 under low temperature treatment 72 h at stamens and pistils differentiation stage
表1 穗分化时期低温处理对不同小麦品种穗粒数的影响
Tab.1 Effects of low temperature stress in different ear differentiation stages on grain number per ear of wheat varieties
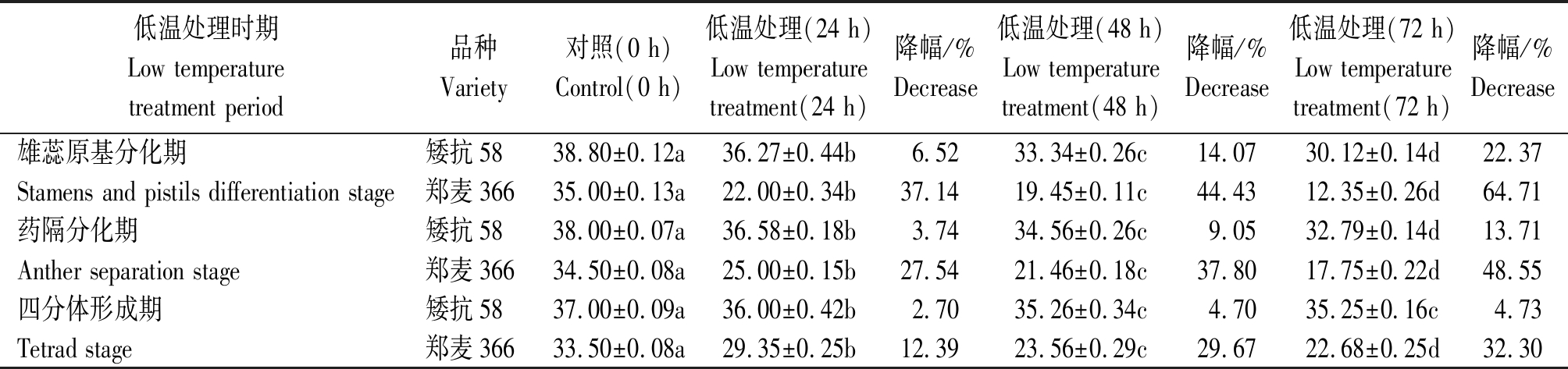
低温处理时期Low temperature treatment period品种Variety对照(0 h)Control(0 h)低温处理(24 h)Low temperature treatment(24 h)降幅/%Decrease低温处理(48 h)Low temperature treatment(48 h)降幅/%Decrease低温处理(72 h)Low temperature treatment(72 h)降幅/%Decrease雄蕊原基分化期矮抗5838.80±0.12a36.27±0.44b6.5233.34±0.26c14.0730.12±0.14d22.37Stamens and pistils differentiation stage郑麦36635.00±0.13a22.00±0.34b37.1419.45±0.11c44.4312.35±0.26d64.71药隔分化期矮抗5838.00±0.07a36.58±0.18b3.7434.56±0.26c9.0532.79±0.14d13.71Anther separation stage郑麦36634.50±0.08a25.00±0.15b27.5421.46±0.18c37.8017.75±0.22d48.55四分体形成期矮抗5837.00±0.09a36.00±0.42b2.7035.26±0.34c4.7035.25±0.16c4.73Tetrad stage郑麦36633.50±0.08a29.35±0.25b12.3923.56±0.29c29.6722.68±0.25d32.30
注:同行数据后不同小写字母表示差异显著(P<0.05)。
Note: Different lowercase letters after data indicate that the difference is significant(P<0.05).
从小麦幼穗发育的3个时期来看,可溶性蛋白质含量随着幼穗发育的推进而不断升高,小麦抗低温能力也越来越强。
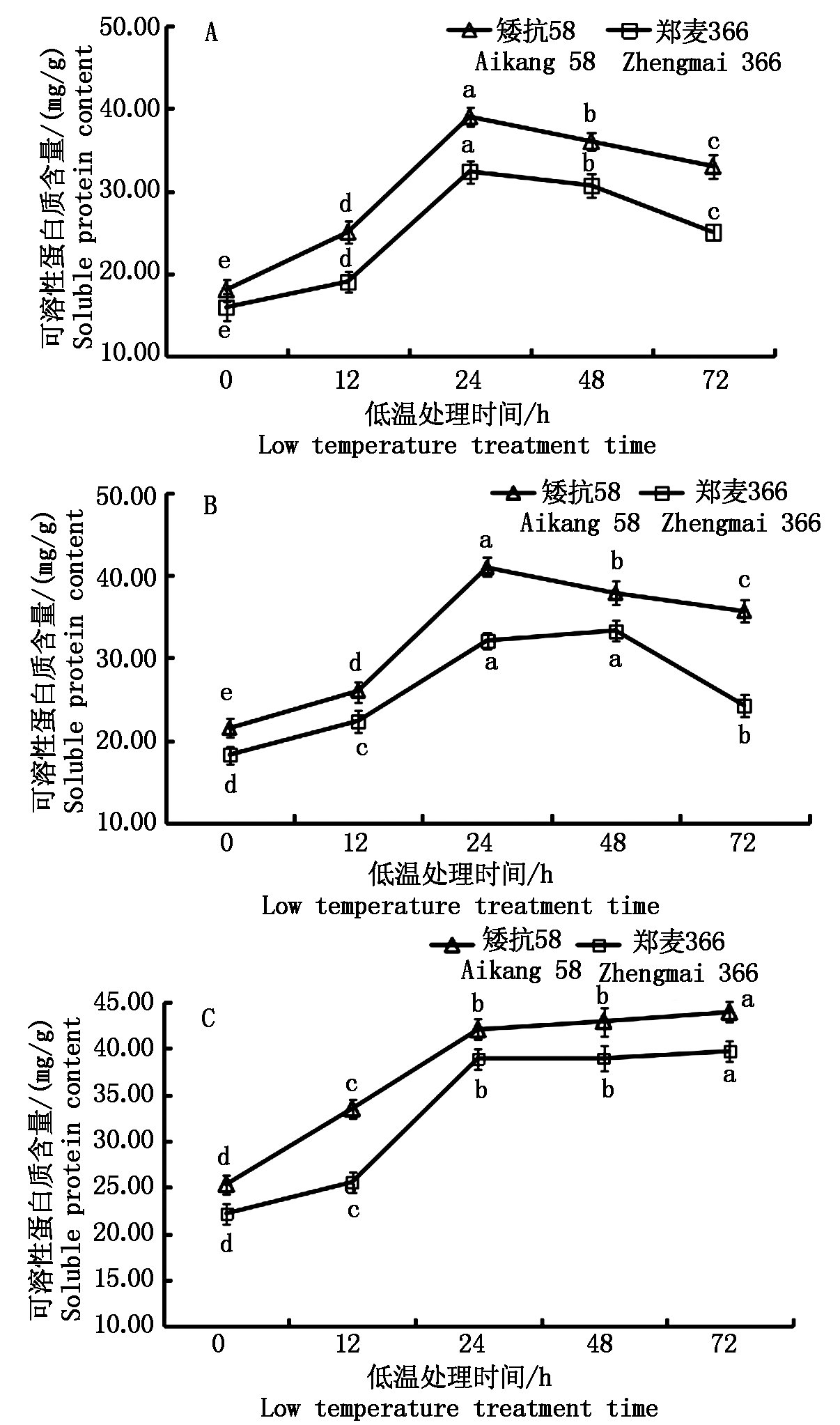
A.雌雄蕊原基分化期;B.药隔分化期;C.四分体形成期。图中不同小写字母表示同一品种不同处理之间差异显著(P<0.05)。图4-8同。
A.Stamens and pistils differentiation stage; B.Anther separation stage; C.Tetrad stage. The different lowercase letters in the figure indicate that the difference between the different treatments of the same variety at the 0.05 level is significant.The same as Fig.4-8.
图3 低温处理对小麦幼穗可溶性蛋白质含量的影响
Fig.3 Effect of low temperature treatment on soluble protein content in young ears of wheat
2.3 低温胁迫对参试材料幼穗可溶性糖含量的影响
由图4可知,雌雄蕊原基分化期、药隔分化期、四分体形成期经低温胁迫后,2个小麦品种可溶性糖含量在0~48 h内均呈显著上升趋势; 48~72 h可溶性糖含量下降显著。随幼穗的发育,2个小麦品种的可溶性糖含量绝对值逐渐升高。
低温处理下,矮抗58的可溶性糖含量均高于郑麦366。2个小麦品种幼穗可溶性糖含量在低温处理的48~72 h均出现了下降趋势,但都高于对照。可溶性糖含量下降的可能原因是随着低温处理时间的延长,植株需要更多的可溶性糖用于抵抗低温的胁迫,可溶性糖的消耗速度大于幼穗产生可溶性糖的速度。

图4 低温处理对小麦幼穗可溶性糖含量的影响
Fig.4 Effect of low temperature treatment on soluble sugar content in young ears of wheat
2.4 低温胁迫对参试材料幼穗POD活性的影响
低温胁迫后2个小麦品种幼穗POD活性均升高,且显著高于对照。不同处理下矮抗58的POD活性均大于郑麦366(图5)。雌雄蕊原基分化期,低温处理0~48 h,矮抗58的POD活性呈一直上升趋势, 48~72 h略有下降;郑麦366在低温处理0~24 h内,POD活性升高,24 h后开始下降(图5-A)。药隔分化期,矮抗58和郑麦366幼穗POD活性变化趋势一致,均在0~24 h升高,24 h后开始下降,但矮抗58幼穗POD活性高于郑麦366,且下降速度小于郑麦366(图5-B)。四分体形成期,矮抗58的POD活性一直处于上升趋势,而郑麦366则在低温处理的0~48 h内,POD活性呈上升趋势,48 h后略有下降(图5-C)。
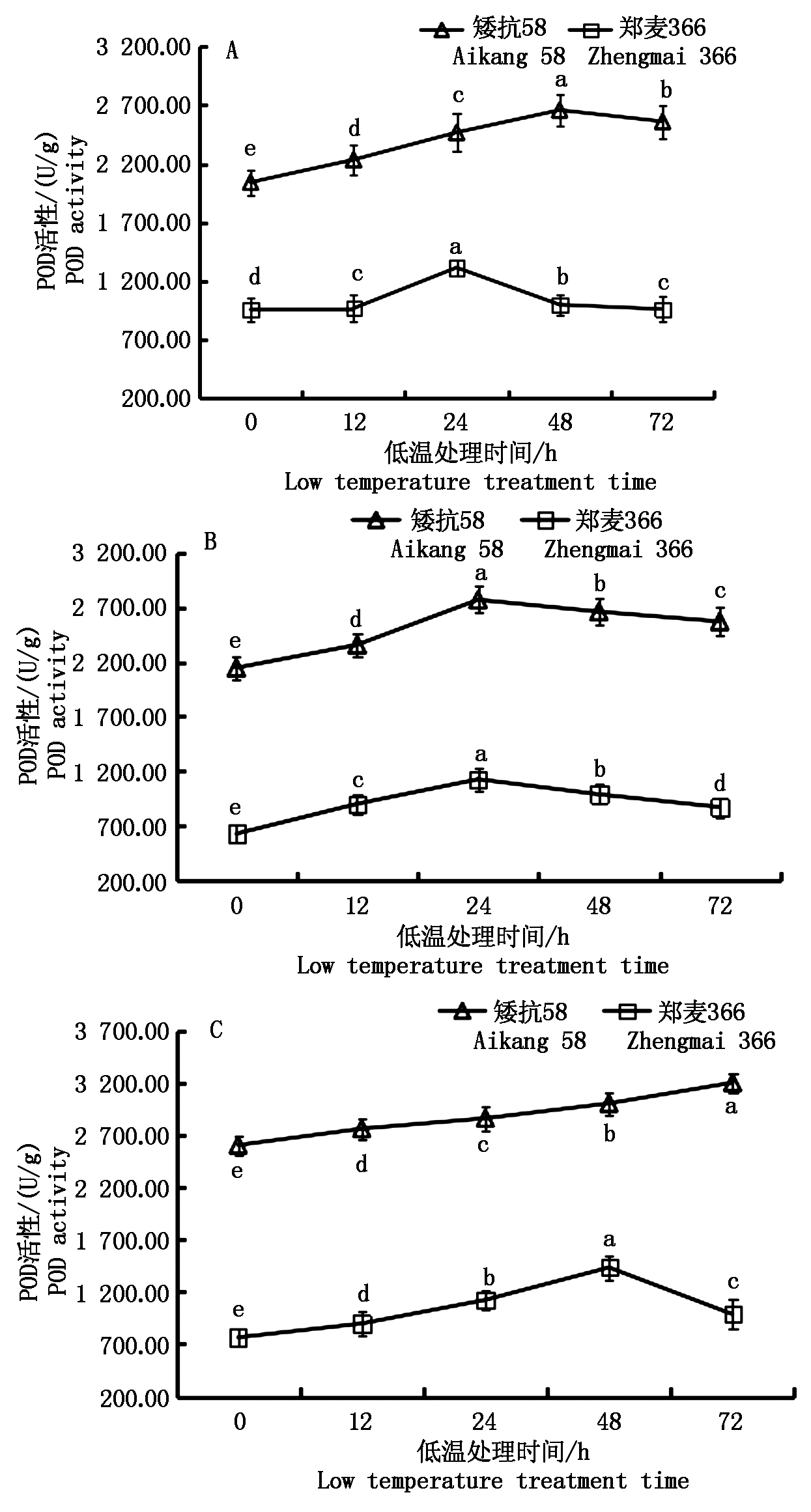
图5 低温处理对小麦幼穗POD活性的影响
Fig.5 Effect of low temperature treatment on POD activity in young ears of wheat
2.5 低温胁迫对参试材料幼穗SOD活性的影响
由图6可知,低温处理后,矮抗58和郑麦366幼穗SOD活性均升高显著;随小麦幼穗发育的推进,矮抗58和郑麦366幼穗SOD活性不断升高,均显著高于对照。
雌雄蕊原基分化期、药隔分化期和四分体形成期,2个小麦品种SOD活性均呈先升高后降低的趋势。雌雄蕊原基分化期,矮抗58在低温处理的24 h,SOD活性迅速达到最大值,24~72 h SOD活性缓慢下降;郑麦366在低温处理的0~48 h,SOD活性一直呈上升趋势,48~72 h SOD活性开始下降(图6-A)。药隔分化期,矮抗58和郑麦366在低温处理的0~24 h,SOD活性迅速上升,之后开始下降。低温处理24 h后,郑麦366幼穗中SOD活性下降幅度明显大于矮抗58(图6-B)。四分体形成期,低温处理下矮抗58的SOD活性变化较平缓,在低温处理的0~48 h SOD活性一直呈上升趋势,48~72 h 稍有下降。郑麦366幼穗中SOD活性在低温处理的0~12 h缓慢上升, 12~24 h迅速上升,24 h达到最大值,处理的24~72 h呈下降趋势(图6-C)。
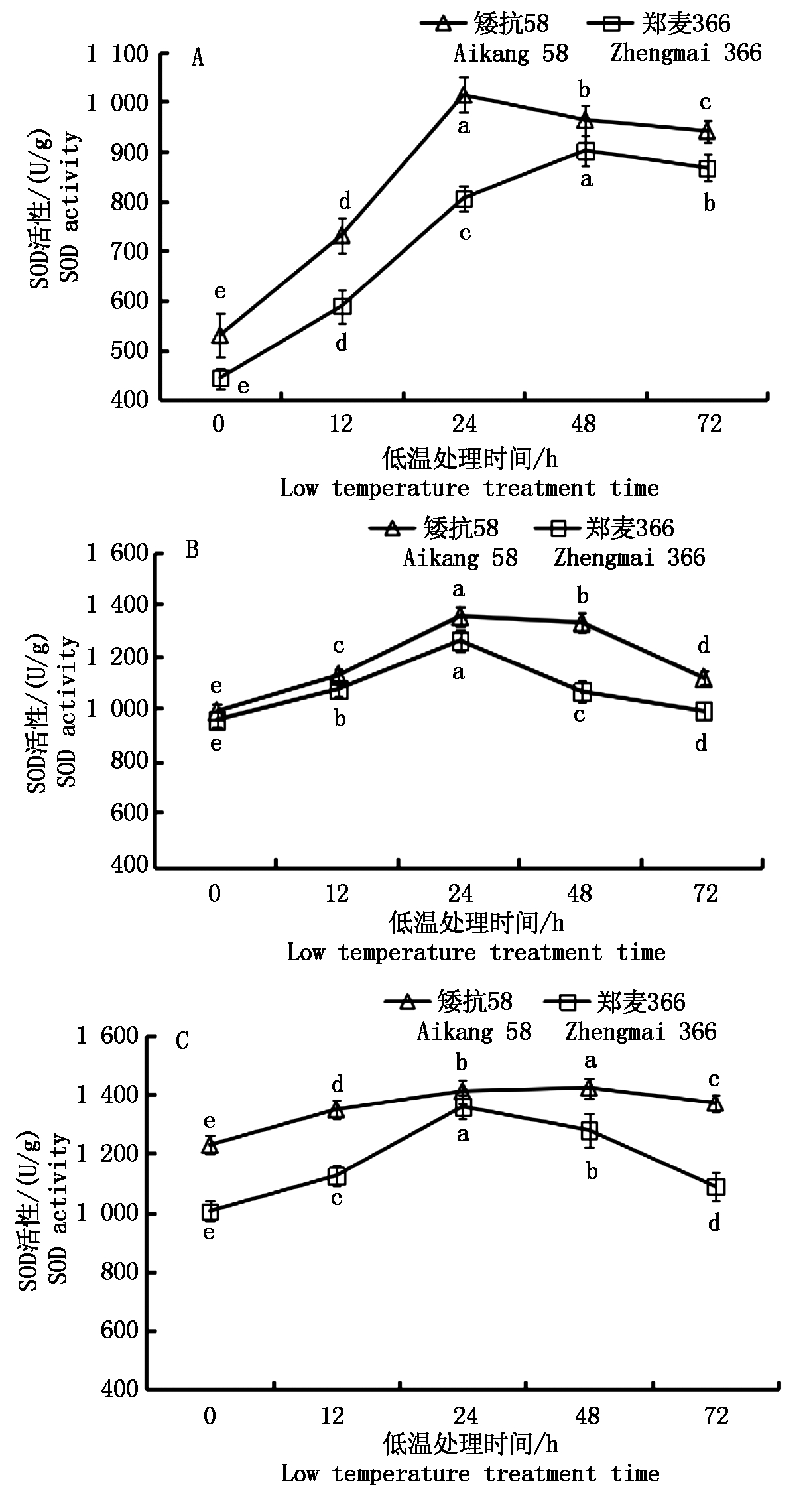
图6 低温处理对小麦幼穗SOD活性的影响
Fig.6 Effect of low temperature treatment on SOD activity in young ears of wheat
2.6 低温胁迫对参试材料幼穗CAT活性的影响
由图7可知,低温处理后2个小麦品种幼穗CAT活性均显著高于对照,并且呈先升高后降低的趋势;随幼穗的发育推进,CAT活性不断升高。
雌雄蕊原基分化期,低温处理0~12 h内,参试材料的CAT活性缓慢升高,12~24 h则迅速升高。矮抗58在低温处理24 h后CAT活性开始缓慢下降,郑麦366则在低温处理48 h后,CAT活性迅速下降(图7-A)。药隔分化期,低温处理0~24 h,2个小麦品种的CAT活性均呈持续升高趋势,24~48 h开始缓慢下降, 48 h后2个小麦品种均下降,但郑麦366下降幅度明显大于矮抗58(图7-B)。四分体形成期低温处理后,2个小麦品种CAT活性变化趋势相同,在0~24 h缓慢上升, 24~72 h缓慢下降(图7-C)。
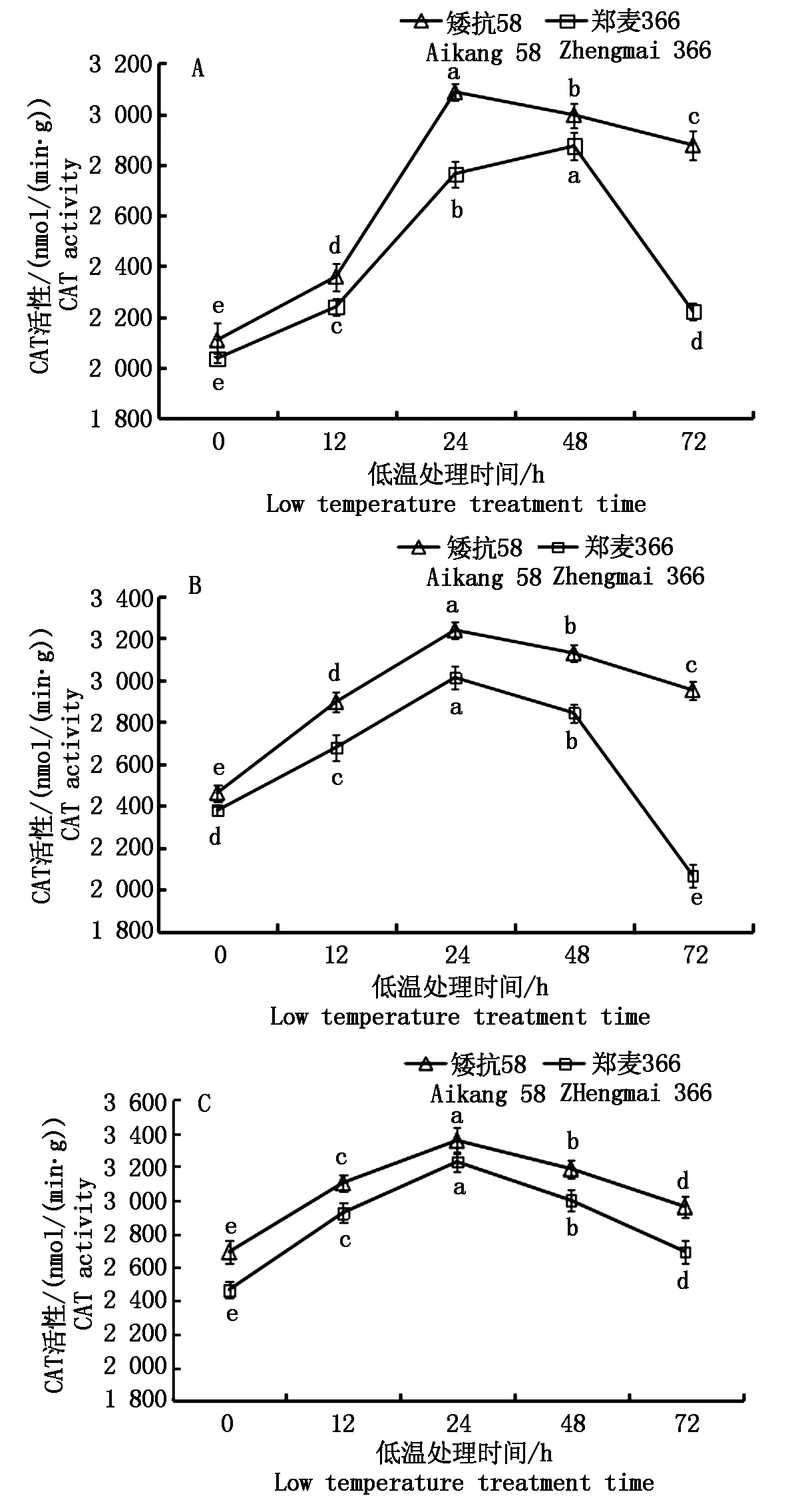
图7 低温处理对小麦幼穗CAT活性的影响
Fig.7 Effect of low temperature treatment on CAT activity in young ears of wheat
2.7 低温胁迫对参试材料幼穗MDA含量的影响
低温胁迫后,2个小麦品种MDA含量均显著高于对照,随处理时间的延长,MDA含量总体呈升高-降低-升高的趋势(图8)。
雌雄蕊原基分化期,低温处理后,矮抗58幼穗中MDA含量小于郑麦366。0~12 h,郑麦366 MDA含量上升幅度大于矮抗58,12~24 h郑麦366 MDA含量开始下降,24 h后又迅速升高。矮抗58在0~12 h内,幼穗中MDA含量有所上升,但上升幅度不大,12~24 h缓慢下降,24 h后开始缓慢上升(图8-A)。药隔分化期低温处理0~72 h内,矮抗58和郑麦366 MDA含量均呈现升高-降低-缓慢升高的趋势,郑麦366的MDA含量始终大于矮抗58(图8-B)。四分体形成期低温处理0~12 h内,矮抗58和郑麦366 MDA含量呈上升趋势,低温处理12 h后,MDA含量变化趋于平稳(图8-C)。

图8 低温处理对小麦幼穗MDA含量的影响
Fig.8 Effect of low temperature treatment on MDA content in young ears of wheat
2.8 小麦穗粒数与生理生化指标的相关性分析
对不同穗分化时期低温胁迫下2个小麦品种结实粒数与生理生化指标进行相关性分析,结果见表2。低温胁迫后,穗粒数与POD活性呈极显著正相关,与MDA含量呈极显著负相关,与SOD活性的相关性也达到显著水平。
表2 低温处理穗粒数与各生理指标的相关性
Tab.2 Correlation of grain number per ear and physiological indices under low temperature
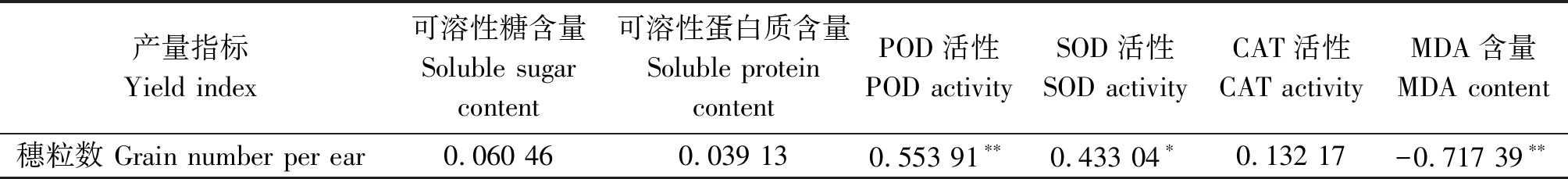
产量指标Yield index可溶性糖含量Soluble sugar content可溶性蛋白质含量Soluble protein contentPOD活性POD activitySOD活性SOD activityCAT活性CAT activityMDA含量MDA content穗粒数 Grain number per ear0.060 460.039 130.553 91∗∗0.433 04∗0.132 17-0.717 39∗∗
注:*表示0.05显著水平;**表示0.01极显著水平。
Note:*means significant difference at 0.05 level;** means significant difference at 0.01 level.
3 结论与讨论
不同小麦品种遭受春季低温危害的差异究竟是其幼穗发育进程差异还是品种自身抗倒春寒能力差异造成的,不同研究者存在不同观点。多数研究者认为,对小麦抗倒春寒的研究需在同一发育进程且在低温敏感期进行鉴定[7-8]。本研究以矮抗58和郑麦366为材料,在保持其他因素一致的情况下,选择幼穗分化相同时期(雌雄蕊原基分化期、药隔分化期、四分体形成期)进行抗倒春寒能力的研究,减少了不同发育时期造成的误差,确保了试验的准确性。研究者对于春季低温胁迫下小麦冻害的敏感时期研究结果不尽相同。刘平湘等[9]、胡新等[10]认为,雌雄蕊分化末期到药隔分化初期可能是小麦冻害的敏感时期;Single[11]认为,开花期为低温敏感期;皇甫自起等[12]认为,小花分化期至药隔形成期前后40多天均为敏感期;曾正兵等[13]认为,药隔期之后为冬小麦幼穗分化低温敏感期。本研究结果表明,矮抗58和郑麦366在雌雄蕊原基分化期、药隔分化期、四分体形成期低温胁迫后,穗粒数均有下降,且随着低温处理时间的延长,穗粒数降幅增大。2个小麦品种在同一处理强度下,随着幼穗发育时期推后,小麦穗粒数降幅逐渐降低。对于不抗倒春寒小麦品种郑麦366来说,雌雄蕊原基分化期对低温最敏感,其次是药隔分化期,四分体形成期也有影响,但影响较小。对于抗倒春寒小麦品种矮抗58来说,雌雄蕊原基分化期、药隔分化期短时间的低温处理对穗粒数的影响不大,但随着低温处理时间的延长,穗粒数降幅有所增加;在四分体形成期,低温处理的0~72 h内对矮抗58的穗粒数影响均不大,说明矮抗58的低温敏感期在雌雄蕊原基分化期,与刘平湘等[9]、胡新等[10]对敏感期的研究结果基本一致,与Single [11]、皇甫自起等[12]、曾正兵等[13]对敏感期的研究结果不同。
可溶性糖、可溶性蛋白质与植物的抗寒性密切相关[14-18],是细胞内重要的渗透调节物质,能增强细胞的持水力从而增强植物的抗寒性。本研究结果表明,在雌雄蕊原基分化期和药隔分化期,2个小麦品种幼穗可溶性蛋白质含量在低温处理的0~24 h内均呈持续增加趋势,可溶性糖含量在低温处理的0~48 h内均呈连续增加趋势。3个幼穗分化时期低温处理下,矮抗58幼穗的可溶性蛋白质和可溶性糖含量均大于郑麦366;总体上随低温处理时间的延长,可溶性蛋白质、可溶性糖含量均出现下降,矮抗58的下降幅度小于郑麦366。下降的原因可能是持续低温使处于低温敏感期的幼穗消耗了更多的可溶性糖和可溶性蛋白质,而新合成可溶性糖和可溶性蛋白质的速率小于消耗的速率。在四分体形成期可溶性蛋白质含量呈上升趋势,说明小麦幼穗在该发育时期能产生更多的可溶性蛋白质以抵御低温胁迫,这与李春燕等[19]对低温胁迫下小麦不同发育时期叶片生理指标的研究结果相似。
低温能诱导植株体内产生过多的超氧阴离子![]() 和过氧化氢(H2O2)等活性氧,并造成清除平衡遭到破坏,引起植株体内活性氧的累积量增加,最终导致植株的损伤或生理机能衰退[20-24]。SOD在降低低温诱发的活性氧数量和减轻低温逆境对植株的伤害中发挥了重要作用[25-27]。SOD、POD分别参与了解除超氧化物、过氧化物的毒害作用[28-29]。SOD能清除植物体内的
和过氧化氢(H2O2)等活性氧,并造成清除平衡遭到破坏,引起植株体内活性氧的累积量增加,最终导致植株的损伤或生理机能衰退[20-24]。SOD在降低低温诱发的活性氧数量和减轻低温逆境对植株的伤害中发挥了重要作用[25-27]。SOD、POD分别参与了解除超氧化物、过氧化物的毒害作用[28-29]。SOD能清除植物体内的![]() 使其发生歧化反应形成O2和H2O2,而对植物有毒害作用的H2O2又被POD和CAT清除,使植株能够免受伤害[30-31]。低温胁迫下小麦穗粒数与生理生化指标相关性分析表明,穗粒数与POD活性呈极显著正相关,与SOD活性呈显著正相关,与MDA含量呈极显著负相关。本试验中,在雌雄蕊原基分化期、药隔分化期,低温处理下,2个小麦品种幼穗的POD、SOD活性均表现先升高后降低的趋势,但是出现最大涨幅及下降的时期不同。在雌雄蕊原基分化期、药隔分化期、四分体形成期低温处理后,矮抗58的POD、SOD活性均高于郑麦366,说明矮抗58在低温胁迫下自身机体能够迅速代谢产生SOD、POD以抵抗低温胁迫。值得注意的是,在雌雄蕊原基分化期、药隔分化期低温胁迫48 h后,矮抗58与郑麦366的POD、SOD、CAT活性均出现了下降趋势,可能是0 ℃持续低温处理对植物造成了不可逆转的伤害,使植物体产生的POD、SOD、CAT活性下降。从低温处理72 h郑麦366的穗粒数严重减少以及膜脂过氧化产物MDA含量持续上升可看出,0 ℃低温处理72 h对植物造成了不可逆转的伤害。
使其发生歧化反应形成O2和H2O2,而对植物有毒害作用的H2O2又被POD和CAT清除,使植株能够免受伤害[30-31]。低温胁迫下小麦穗粒数与生理生化指标相关性分析表明,穗粒数与POD活性呈极显著正相关,与SOD活性呈显著正相关,与MDA含量呈极显著负相关。本试验中,在雌雄蕊原基分化期、药隔分化期,低温处理下,2个小麦品种幼穗的POD、SOD活性均表现先升高后降低的趋势,但是出现最大涨幅及下降的时期不同。在雌雄蕊原基分化期、药隔分化期、四分体形成期低温处理后,矮抗58的POD、SOD活性均高于郑麦366,说明矮抗58在低温胁迫下自身机体能够迅速代谢产生SOD、POD以抵抗低温胁迫。值得注意的是,在雌雄蕊原基分化期、药隔分化期低温胁迫48 h后,矮抗58与郑麦366的POD、SOD、CAT活性均出现了下降趋势,可能是0 ℃持续低温处理对植物造成了不可逆转的伤害,使植物体产生的POD、SOD、CAT活性下降。从低温处理72 h郑麦366的穗粒数严重减少以及膜脂过氧化产物MDA含量持续上升可看出,0 ℃低温处理72 h对植物造成了不可逆转的伤害。
综上所述,在雌雄蕊原基分化期、药隔分化期、四分体形成期,随着低温处理时间的延长,2个小麦品种的幼穗受到不同程度的损伤。随着幼穗发育的推进,小麦幼穗的抗低温能力逐渐增强。不同时期低温处理下,从雌雄蕊原基分化期到四分体形成期小麦穗粒数逐渐增多。说明雌雄蕊原基分化期是幼穗低温最敏感时期。生理特性研究结果表明,抗春季低温胁迫小麦品种较低温敏感品种的幼穗膜脂过氧化程度轻,积累的渗透调节物质较多,抗氧化酶活性较强,小麦幼穗通过多种方式协调来适应春季低温胁迫,小麦抗春季低温在生理指标上表现出多方面的综合防御。综合穗粒数、生理指标变化及相关性分析,可以把POD、SOD活性和MDA含量作为小麦幼穗抗倒春寒特性的生理评价指标。
[1] 李茂松,王道龙,张强,迟永刚,王春艳,渡边好昭,吉田久.2004-2005年黄淮海地区冬小麦冻害成因分析[J].自然灾害学报,2005,14(4):51-55. doi:10.13577/j.jnd.2005.0409.
Li M S, Wang D L, Zhang Q, Chi Y G, Wang C Y, Kiribuchi-Otobe C, Yoshida H. Cause analysis of frost damage to winter wheat in Huang-Huai-Hai plain during 2004-2005[J]. Journal of Natural Disasters, 2005,14(4):51-55.
[2] 郭天财,王永华.小麦高产与防灾减灾技术[M].郑州:中原农民出版社,2016:66-68.
Guo T C, Wang Y H. Techniques for high yield and disaster prevention and reduction of wheat [M].Zhengzhou: Zhongyuan Farmers Press, 2016:66-68.
[3] 孙苗苗,王志强,高翔,龚璞,辛泽毓,林同保.河南主推小麦品种对低温胁迫的生理响应及耐寒性分析[J].麦类作物学报,2016,36(3):316-324. doi:10.7606/j.issn.1009-1041.2016.03.09.
Sun M M, Wang Z Q, Gao X, Gong P, Xin Z Y, Lin T B. Cold tolerance evaluation of wheat varieties in henan based on their physiological response to low temperature stress[J]. Journal of Triticeae Crops, 2016,36(3):316-324.
[4] 康国章,岳彩凤,彭慧芳,韩巧霞,李鸽子,许薇,刘国芹,郭天财.冻害胁迫对小麦叶片抗寒生理生化指标的影响[J]. 河南农业科学,2011,40(12):56-60. 10.15933/j.cnki.1004-3268.2011.12.025.
Kang G Z, Yue C F, Peng H F, Han Q X, Li G Z, Xu W, Liu G Q, Guo T C. Effects of freezing stress on anti-chilling contents in wheat leaves[J].Journal of Henan Agricultural Sciences, 2011,40(12):56-60.
[5] 潘晓云,曹琴东,王根轩.膜脂过氧化作为扁桃品种抗寒性鉴定指标研究[J].生态学报,2002,22(11):1902-1911. doi:10.3321/j.issn:1000-0933.2002.11.015.
Pan X Y, Cao Q D, Wang G X. Evaluation of lipid peroxidation for use in selection of cold hardiness cultivars of almond[J]. Acta Ecologica Sinica, 2002, 22(11):1902-1911.
[6] 崔金梅,郭天财.小麦的穗[M].北京:中国农业出版社,2010:35-38.
Cui J M, Guo T C. The ear of wheat[M].Beijing: China Agriculture Press, 2010:35-38.
[7] 刘大同,邢潇悦,李东升,胡文静,程晓明,高德荣,程顺和.小麦穗分化不同阶段对低温胁迫的生理响应[J].江苏农业学报, 2017,33(6):1212-1219. doi:10.3969/j.issn.1000-4440.2017. 06.003.
Liu D T, Xing X Y, Li D S, Hu W J, Cheng X M, Gao D R, Cheng S H. The physiological response to low temperature of wheat during young spike development[J].Jiangsu Journal of Agricultural Sciences, 2017, 33(6):1212-1219.
[8] 钟秀丽,王道龙,吉田久,胡新,赵鹏,韩立帅,王晓光,黄绍华,黄建英,孙忠富. 冬小麦品种抗霜冻力的影响因素分析[J].作物学报, 2007,33(11):1810-1814. doi:10.3321/j.issn:0496-3490.2007.11.011.
Zhong X L, Wang D L,Ji T J,Hu X,Zhao P,Han L S,Wang X G,Huang S H,Huang J Y,Sun Z F. Analysis on the factors affecting frost resistance for winter wheat[J].Acta Agronomica Sinica, 2007,33(11):1810-1814.
[9] 刘平湘,郭天财,韩巧霞,王永华,吴晓. 不同类型冬小麦品种抗晚霜冻能力的鉴定[J]. 中国农学通报,2010,26(19):94-98.
Liu P X,Guo T C,Han Q X,Wang Y H,Wu X.The evaluation of frost resistance of different type winter varieties[J].Chinese Agricultural Science Bulletin, 2010,26(19):94-98.
[10] 胡新,黄绍华,黄建英,肖召杰.晚霜冻害与小麦品种的关系:1998年霜冻害调查报告之一[J].中国农业气象,1999,20(3):34-48.
Hu X,Huang S H,Huang J Y,Xiao Z J. Influence of late frost on different wheat cultivars: The first report of investigation on late frost injury to wheat in 1998[J].Chinese Journal of Agrometeorology,1999,20(3):34-48.
[11] Single W V. Frost injury and the physiology of the wheat plant [J]. Journal of the Australian Institute of Agricultural Science, 1984,17:128-134.
[12] 皇甫自起,常守乾,李秀华,陈德华,晏中峰.豫东地区小麦冻害调查分析[J].河南农业科学,1996(4):3-6. doi:10.15933/j.cnki.1004-3268.1996.04.001.
Huangpu Z Q,Chang S Q, Li X H, Chen D H,Yan Z F. Investigation and analysis of wheat freezing injury in eastern Henan Province[J]. Journal of Henan Agricultural Sciences, 1996(4):3-6.
[13] 曾正兵,钟秀丽,王道龙,郭金耀,赵鹏,王晓光,韩立帅.冬小麦拔节后幼穗低温敏感期的鉴定[J].自然灾害学报,2006,15(6):297-300.
Zeng Z B,Zhong X L,Wang D L,Guo J Y,Zhao P,Wang X G,Han L S.Identification of young ear′s low temperature sensitive phase after jointing stage of winter wheat[J].Journal of Natural Disasters, 2006, 15(6):297-300.
[14] 李波,方志坚. 耐低温玉米自交系的筛选及其叶片生理特性和细胞结构变化[J]. 河南农业科学,2018,47(10):31-37.doi:10:15933/j.cnki.1004-3268.2018.10.006.
Li B, Fang Z J. Screening of low-temperature tolerance maize inbred and analysis of leaf physiological characteristics and cellular structure changes [J]. Journal of Henan Agricultural Sciences, 2018,47(10):31-37.
[15] Gomes F P, Oliva M A, Mielke M S, Almeida A A F, Aquinob L A. Osmotic adjustment, proline accumulation and cell membrane stability in leaves of Cocos nucifera submitted to drought stress[J].Scientia Horticulturae,2010,126(3):379-384. doi:10.1016/j.scienta.2010.07.036.
[16] Terzioglu S, Ekmekci Y.Variation of total soluble seminal root proteins of tetraploid wild and cultivated wheat induced at cold acclimation and freezing[J]. Acta Physiologiae Plantarum, 2004, 26(4):443-450. doi:10.1007/s11738-004-0035-6.
[17] 陈璇,李金耀,马纪,张富春.低温胁迫对春小麦和冬小麦叶片游离脯氨酸含量变化的影响[J]. 新疆农业科学,2007,44(5):553-556.doi:10.3969/j.issn.1001-4330.2007.05.002.
Chen X, Li J Y, Ma J, Zhang F C. Effect of low temperature stress on change of free proline content in the leaves of spring and winter wheat[J]. Xinjiang Agricultural Sciences, 2007, 44(5):553-556.
[18] 王树刚,王振林,王平,王海伟,李府,黄玮,武玉国,尹燕枰.不同小麦品种对低温胁迫的反应及抗冻性评价[J].生态学报,2011,31(4):1064-1072.
Wang S G, Wang Z L, Wang P, Wang H W, Li F, Huang W, Wu Y G, Yin Y P. Evaluation of wheat freezing resistance based on the responses of the physiological indices to low temperature stress[J]. Acta Ecologica Sinica, 2011,31(4):1064-1072.
[19] 李春燕,徐雯,刘立伟,雷晓伟,杨景,周冬冬,朱新开,郭文善.药隔至开花期低温对小麦产量和生理特性的影响[J].麦类作物学报,2016, 36(1):77-85.doi:10.7606/j.issn.1009-1041. 2016. 01.11.
Li C Y, Xu W, Liu L W, Lei X W, Yang J, Zhou D D, Zhu X K, Guo W S. Effect of short-time low temperature from anther connective stage to anthesis on wheat yield and physiological characteristics[J].Journal of Triticeae Crops, 2016,36(1):77-85.
[20] Allen R D. Dissection of oxidative stress tolerance using transgenic plants[J]. Plant Physiology, 1995, 107:1049-1054. doi:10.1104/pp.107.4.1049.
[21] Cakmak I, Marschner H. Magnesium deficiency and high light intensity enhance activities of superoxide dismutase, ascorbate peroxidate and glutathione ruductase in bean leaves[J]. Plant Physiology, 1992,98:1222-1227. doi:10.1104/pp.98.4.1222.
[22] Mehdy M C. Active oxygen species in plant defense against pathogens[J]. Plant Physiology,1994,105(2): 467-472. doi:10.0000/PMID12232215.
[23] Asada K. The water-water cycle in chloroplasts: Scavenging of active oxygens and dissipation of excess photons[J].Annual Review of Plant Physiology and Plant Molecular Biology,1999,50:601-639.doi: 10.1146/annurev.arplant.50.1.601.
[24] Wang P, Duan W, Takabayashi A, Endo T, Shikanai T, Ye J Y, Mi H. Chloroplastic NAD(P)H dehydrogenase in tobacco leaves functions in alleviation of oxidative damage caused by temperature stress[J]. Plant Physiology, 2006, 141(2): 465-474. doi:10.1104/pp.105.070490.
[25] Karpinska B, Karlsson M, Schinkel H, Streller S, Süss K H, Melzer M, Wingsle G. A novel superoxide dismutase with a high isoelectric point in higher plants. Expression, regulation, and protein localization[J]. Plant Physiology, 2001, 126(4): 1668-1677. doi:10.1104/pp.126.4.1668.
[26] Bowler C, van Montague M, Inzé D. Superoxide dismutase and stress tolerance[J]. Annual Review of Plant Physiology and Plant Molecular Biology, 1992, 43(1): 83-116. doi: 10.1146/annurev.arplant.43.1.83.
[27] Chu C C, Lee W C, Guo W Y, Pan S M, Chen L J, Li H M, Jinn T L. A copper chaperone for superoxide dismutase that confers three types of copper/zinc superoxide dismutase activity in Arabidopsis[J]. Plant Physiology, 2005, 139(1): 425-436.doi:10.1104/pp.105.065284.
[28] Noctor G, Fover C H. Ascorbate and glutathione: keeping active oxygen under control [J]. Annu Rev Plant Mol Biol, 1998, 49: 249-279.doi: 10.1146/annurev.arplant.49.1.249.
[29] Blum A, Ebercon A. Cell membrane stability as a measure of drought and heat tolerance in wheat [J]. Crop Science, 1981, 21(1): 43-47. doi:10.2135/cropsci1981.0011183X002100010013x.
[30] Gong J R, Zhao A, Zhang L X, Zhang X S. A comparative study on anti-oxidative ability of several desert plants under drought stress[J]. Acta Botanical Boreali-Occidentalia Sinica, 2004, 24(9): 1570-1577. doi:10.1088/1009-0630/6/5/011.
[31] 曲涛, 南志标. 作物和牧草对干旱胁迫的响应及机理研究进展[J]. 草业学报, 2008, 17(2): 126-135.doi: 10.3321/j.issn:1004-5759.2008.02.018
Qu T, Nan Z B. Research progress on response and mechanisms of crop and grass under drought stress[J]. Acta Prataculturae Sinca, 2008, 17(2): 126-135.