越橘亦称蓝莓,是杜鹃花科(Ericaceae)越橘属(Vaccinium spp.)植物,为多年生常绿或落叶灌木树种。越橘作为我国一种新兴的果树作物之一,栽植面积和产量稳步增长[1-2]。低温是影响植株生长发育、品质和产量形成的主要环境因子之一[3-8],早春低温和晚春霜冻对越橘直接造成伤害或间接影响开花和坐果[9-10]。因此,探讨越橘抗寒机制,提高抗低温能力,对越橘抗寒育种具有重要意义。
不同果树品种抗低温能力差异较大,抗寒能力遗传改良是减轻低温伤害的有效途径之一。越橘不同品种抗低温能力差异较大,其对低温耐受能力主要取决于低温驯化程度[11]。在植物低温驯化过程中,细胞组织结构和基因表达水平均会发生变化[12]。ICE1-CBF-COR低温应答通路是植物响应低温胁迫的主要信号传导模块之一[13-14]。ICE1(Inducer of CBF3 expression 1)为bHLH(Basic helix-loop-helix)转录因子家族成员之一。在拟南芥中,AtICE1结合AtCBF3(CRT binding factor 3)启动子MYC结合位点(CANNTG),激活AtCBF3的表达,AtCBF3促进AtCOR(Cold-regulated)的转录,使拟南芥响应低温胁迫[15]。ICE1基因突变可抑制CBF3的表达,并削弱植株对低温的抗性;超表达ICE1可显著提高转基因植株低温抗性[16-17]。
作为多年生木本植物越橘,低温对树体生长具有重要影响。目前关于ICE1基因调控植物低温抗性已有大量研究,但主要集中在水稻[18]、葡萄[19]和小麦[20]等物种中,越橘中ICE1同源基因的研究尚无报道。本研究以抗寒性较强的越橘品种北陆为试材,分离并鉴定1个越橘ICE1基因VcICE1,通过表达模式、转基因分析及瞬时表达试验,探讨VcICE1在调节越橘响应低温信号过程中的作用,为越橘抗寒育种提供背景资料。
1 材料和方法
试验于2017年5月-2018年9月在中国农业科学院果树研究所、农业部园艺作物种质资源利用重点实验室和山东农业大学作物生物学国家重点实验室进行。
1.1 试验材料
试验所用的植物材料为5年生越橘品种北陆(Vaccinium corymbosum Northland)及其组培苗、野生型拟南芥(Arabidopsis thaliana)和本氏烟草(Nicotiana benthamiana)。
将长势一致的越橘组培苗置于每天16 h光照,光强2 500~3 000 lx,温度为4 ℃的光照培养箱中,分别在处理0,1,8,16,32 h后取叶片,液氮速冻后-70 ℃保存备用。对照置于光照培养箱中25 ℃条件下。野生型拟南芥用来遗传转化。本氏烟草用来进行瞬时表达试验。
1.2 基因克隆和序列分析
以拟南芥AtICE1和苹果MdICE1基因为参考序列,在越橘转录组数据库[21]中进行Blast检索。以越橘叶片的cDNA为模板,根据检索到的序列设计引物VcICE1-F/R扩增开放读码框序列(ORF)。PCR反应程序为:98 ℃预变性 3 min;98 ℃变性10 s,57 ℃退火30 s,72 ℃延伸2 min,30个循环;72 ℃延伸10 min。PCR产物用1.2%琼脂糖凝胶电泳并回收目的条带,连接到克隆载体pEAST blunt zero进行测序。所用的引物序列见表1。
利用软件Weblogo 3(http://weblogo.threeplusone.com)分析VcICE1蛋白的保守序列。利用软件Mega 6.0(http://www. megasoftware.net)引入多个拟南芥bHLH蛋白,对VcICE1 蛋白进行聚类分析。
1.3 RNA的提取与实时荧光定量qRT-PCR分析
分别提取越橘植株的根、1年生枝条(顶部第一和二节间部分)、幼叶、花(盛花期)和成熟果实(花后60 d)的总RNA,总RNA提取采用TaKaRa公司植物总RNA提取试剂盒(Code No.9769)。以总RNA为模板,使用MBI公司反转录试剂盒(Code No.K1621)合成cDNA。首先用普通PCR进行序列验证,目的基因经PCR扩增、回收后,进行测序。基因序列确定后,进行实时荧光定量qRT-PCR分析。内参基因为VcGAPDH。在拟南芥中,以AtUBQ10作为内参基因。仪器为Bio-Rad公司CFX Connect PCR system,试剂为ThermoFisher公司PowerUpTM SYBR Green Master Mix(Code No.A25742)。反应体系:SYBR Mixture 10.0 μL,cDNA 2.0 μL,上下游引物各0.5 μL,加去离子水至20 μL。PCR反应程序:95 ℃预变性2 min;95 ℃变性15 s,58 ℃退火15 s,72 ℃延伸1 min,40个循环;每次循环第2步进行荧光采集。最后采用2-ΔΔCT法分析定量数据。所有PCR都设3次重复。实时荧光定量qRT-PCR引物见表1。
1.4 拟南芥转化和鉴定
构建VcICE1-pRI101过量表达载体,并将其转化农杆菌GV3101,利用农杆菌侵染花序法转化野生型拟南芥。在含有卡那霉素的MS固体培养基上筛选转基因植株。将抗性苗移栽至基质中并放入光照培养箱中进行培养。纯合转基因株系用于试验。具体参照Clough和Bent等[22]方法。载体构建时使用的引物见表1。
表1 所使用的引物
Tab.1 Primers used in this study
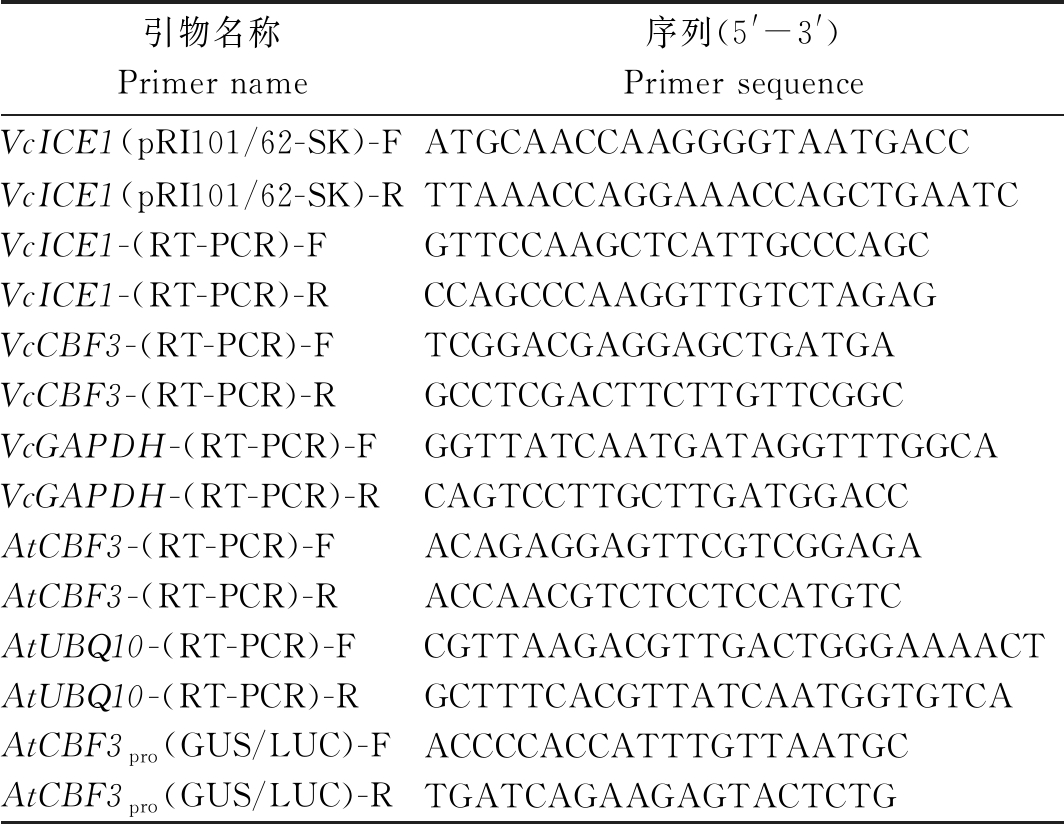
引物名称Primer name序列(5'-3')Primer sequenceVcICE1(pRI101/62-SK)-FATGCAACCAAGGGGTAATGACCVcICE1(pRI101/62-SK)-RTTAAACCAGGAAACCAGCTGAATCVcICE1-(RT-PCR)-FGTTCCAAGCTCATTGCCCAGCVcICE1-(RT-PCR)-RCCAGCCCAAGGTTGTCTAGAGVcCBF3-(RT-PCR)-FTCGGACGAGGAGCTGATGAVcCBF3-(RT-PCR)-RGCCTCGACTTCTTGTTCGGCVcGAPDH-(RT-PCR)-FGGTTATCAATGATAGGTTTGGCAVcGAPDH-(RT-PCR)-RCAGTCCTTGCTTGATGGACCAtCBF3-(RT-PCR)-FACAGAGGAGTTCGTCGGAGAAtCBF3-(RT-PCR)-RACCAACGTCTCCTCCATGTCAtUBQ10-(RT-PCR)-FCGTTAAGACGTTGACTGGGAAAACTAtUBQ10-(RT-PCR)-RGCTTTCACGTTATCAATGGTGTCAAtCBF3pro(GUS/LUC)-FACCCCACCATTTGTTAATGCAtCBF3pro(GUS/LUC)-RTGATCAGAAGAGTACTCTG
1.5 烟草叶片进行瞬时表达试验
把含有ICE1蛋白结合位点,长度为1 128 bp的AtCBF3启动子序列连接到pCAMBIA1301-GUS载体(AtCBF3pro-GUS),转化农杆菌GV3101,与VcICE1-pRI101共注射烟草叶片。注射3 d后的烟草叶片用于GUS活性分析。称取1 g叶片,使用GUS提取缓冲液(50 mmol/L NaH2PO4 pH值7.0,10 mmol/L EDTA,0.1% Triton X-100,0.1% 十二烷基氨酸钠)提取叶片蛋白。使用康为世纪公司的BCA蛋白定量试剂盒(Code No. CW0014)对GUS蛋白进行定量。具体参照Yin等[23]和Jefferson等[24]方法。
将VcICE1的ORF序列重组到pGreenⅡ62-SK载体(VcICE1-pGreenⅡ62-SK),将AtCBF3的启动子序列重组到pGreenⅡ0800-LUC载体(AtCBF3pro-LUC),转化农杆菌GV3101,注射烟草叶片。具体参照An等[25]试验方法。使用活体成像系统检查荧光强度。
1.6 统计分析
使用SPSS软件进行差异显著性分析。不同字母代表差异显著(P﹤0.05)。每个试验重复3次。
2 结果与分析
2.1 越橘VcICE1的克隆、蛋白质结构域和系统进化分析
通过RT-PCR技术获得长度约为1 600 bp的条带。对所得片段测序分析,结果显示,VcICE1的ORF长度为1 566 bp,编码含有522个氨基酸的蛋白质。使用Weblogo 3分析VcICE1及其他植物ICE1蛋白的保守结构域。结果表明,VcICE1含有1个保守的bHLH结构域,属于bHLH转录因子家族(图1)。将VcICE1与拟南芥bHLH蛋白进行系统进化分析,发现VcICE1与AtICE1(AtbHLH116)的同源性最高(图2)。因此命名为VcICE1,GenBank登录号为MH717245。
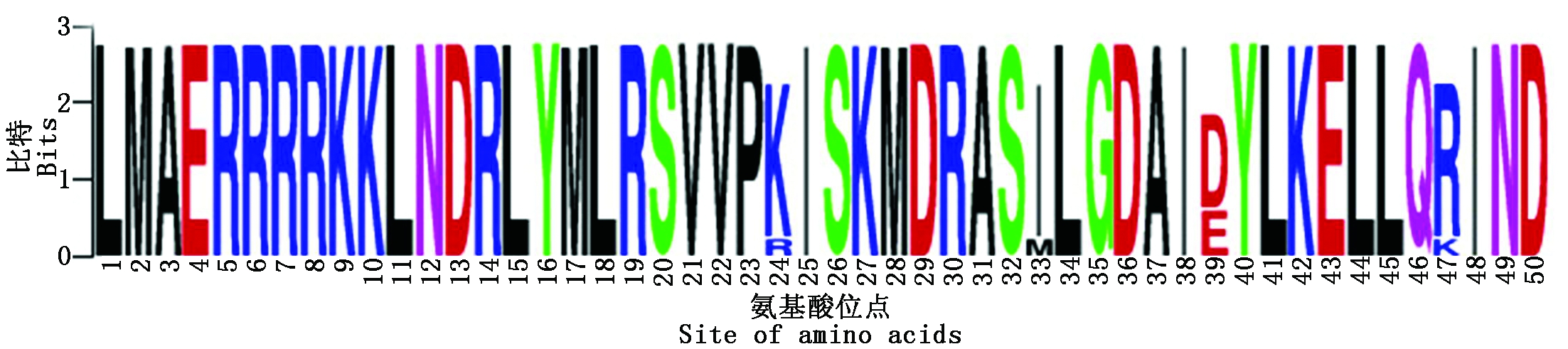
图1 越橘VcICE1蛋白与其他植物ICE1蛋白的bHLH保守结构域序列标签
Fig.1 The conserved domain logo of the VcICE1 in blueberry and other plant ICE1 protein
2.2 VcICE1和VcCBF3基因表达的分析
利用实时荧光定量qRT-PCR分析VcICE1和VcCBF3(GenBank登录号为FJ222601)在越橘不同组织器官中的表达情况。结果显示,VcICE1在幼叶中表达量最高,在果实中表达量最低。VcCBF3主要在幼叶中表达,其次在花中,在根、枝条和果实中表达均较低(图3)。分析VcICE1和VcCBF3对低温的响应。结果表明,在低温处理后1,8,16,32 h,与低温处理0 h比较,VcICE1的表达量分别提高3.00,4.30,6.52,10.26倍,VcCBF3的表达量分别提高1.63,4.78,5.54,8.65倍。随低温处理时间的增加,这2个基因的表达量均表现持续上调,在处理后32 h达到最高(图4)。
2.3 异位表达VcICE1提高转基因拟南芥低温抗性
为进一步研究VcICE1的功能,构建该基因的植物超量表达载体。将重组表达载体转化农杆菌GV3101,通过农杆菌介导的遗传转化侵染野生型拟南芥。实时荧光定量qRT-PCR检测转基因株系中VcICE1和AtCBF3的表达量,结果获得OE-1和OE-2 2个转基因株系,转基因株系中VcICE1和AtCBF3的表达量均显著高于野生型(图5-A)。正常温度(22 ℃)条件下,野生型与转基因植株的根长无明显差异。低温(4 ℃)处理后,2个转基因拟南芥株系的根长分别为2.96,3.21 cm,野生型为1.81 cm,2个转基因株系的根长分别为野生型的1.63,1.77倍。转基因植株的根长显著高于野生型,说明超表达VcICE1可能提高了转基因植株的低温抗性(图5-B-C)。
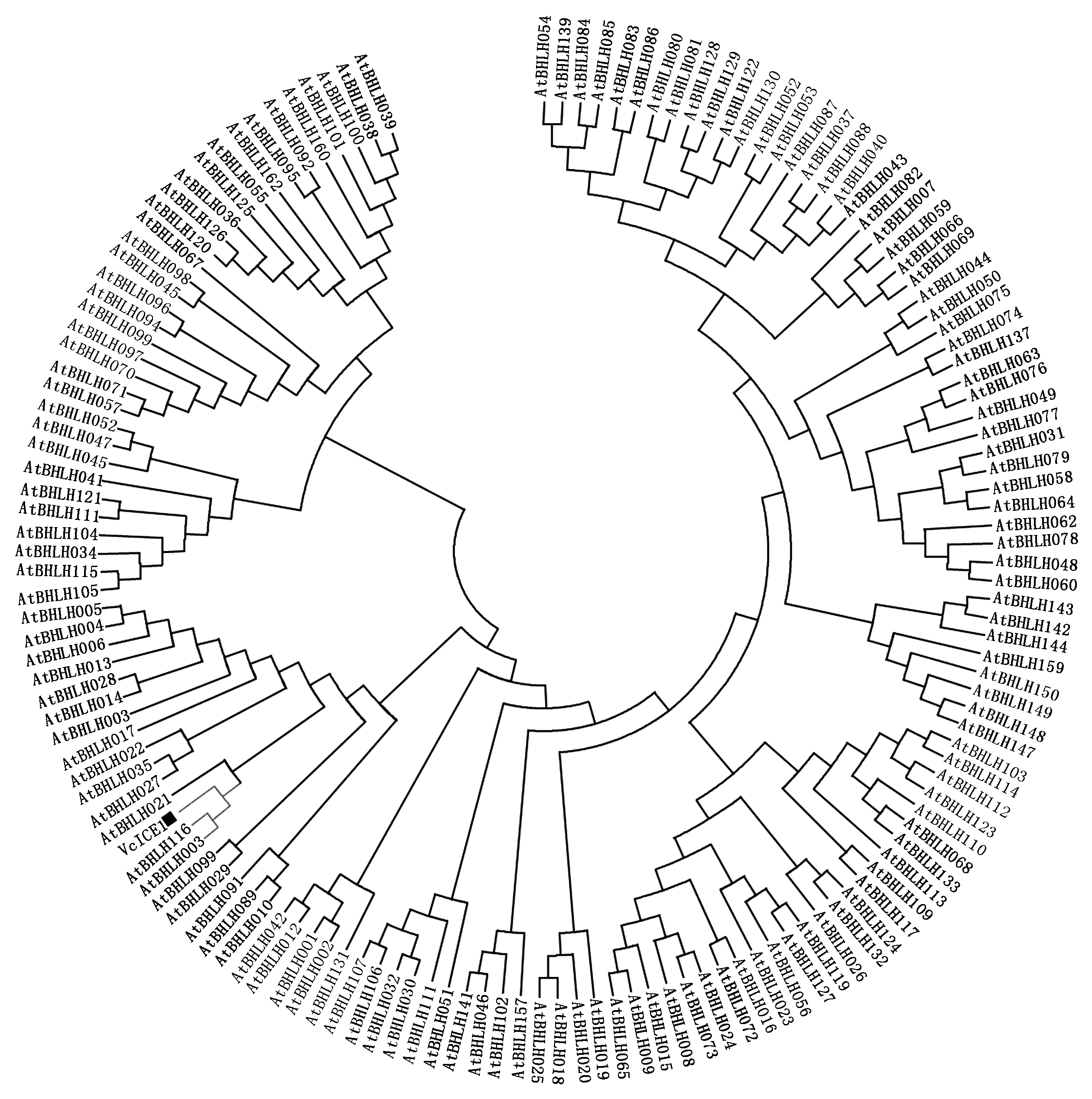
图2 越橘VcICE1和拟南芥bHLH蛋白系统进化分析
Fig.2 Phylogenetic relationships of VcICE1 of blueberry and bHLH protein of Arabidopsis thaliana
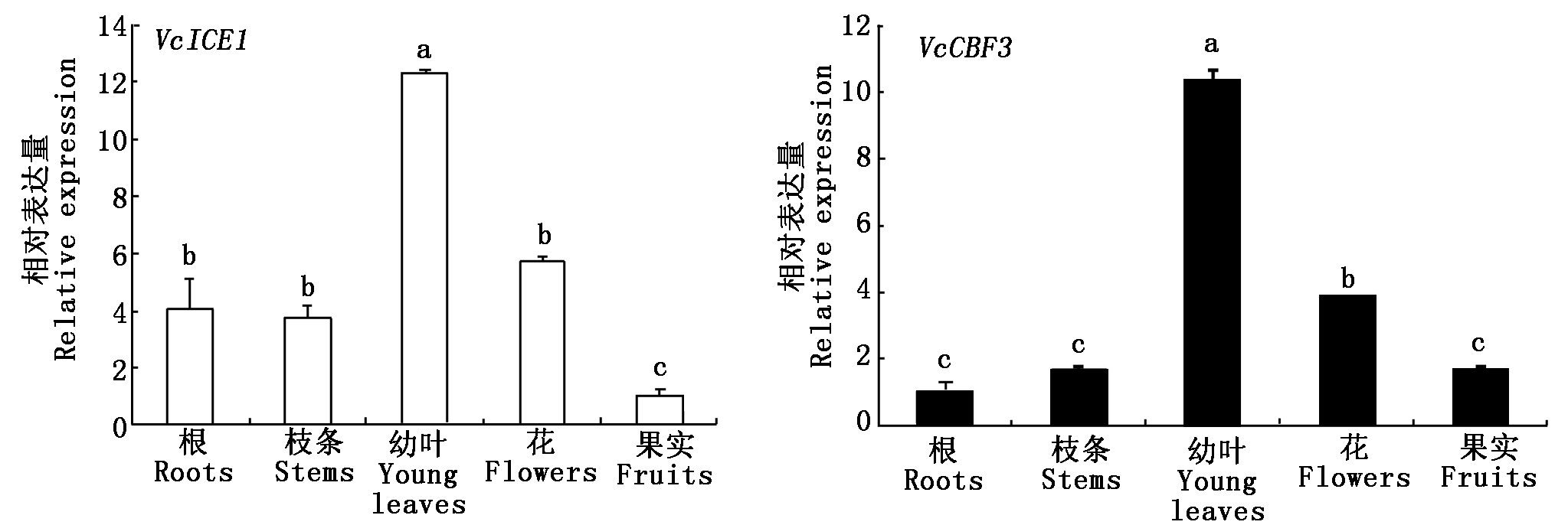
不同小写字母表示差异显著(P﹤0.05)。图4-7同。
Different lowercase letter indicate significant differences(P﹤0.05). The same as Fig.4-7.
图3 不同组织器官中VcICE1和VcCBF3转录水平的表达分析
Fig.3 Expression of VcICE1 and VcCBF3 in different organs of blueberry
2.4 VcICE1促进AtCBF3的表达
为研究VcICE1对冷信号途径中其下游基因CBF3的调控作用,构建VcICE1-pGreenⅡ62-SK和AtCBF3pro-LUC载体,以及VcICE1-pRI101和AtCBF3pro-GUS载体,并在烟草叶片中瞬时表达。如图6所示,共转VcICE1和AtCBF3启动子的叶片中,其相对荧光强度显著高于对照,是对照的3.23倍。共转叶片中相对GUS活性也显著高于对照,为3.88倍(图7)。说明VcICE1可促进AtCBF3的表达。
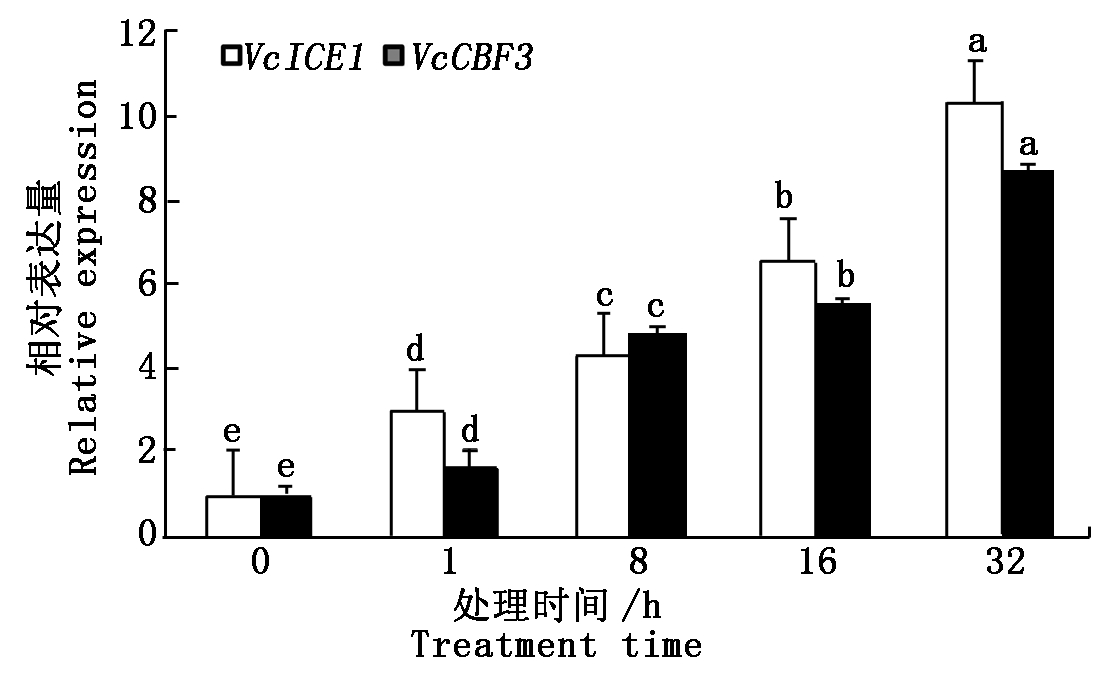
图4 VcICE1和VcCBF3对低温的响应
Fig.4 Expression of VcICE1 and VcCBF3 genes in response to cold
3 结论与讨论
低温是严重影响植物生长发育、果实品质和产量的要素之一。探讨植物抗低温能力,是多年来农业领域的重点和热点问题。阐明植物抗低温胁迫的作用机制,具有重要的理论和实践意义。
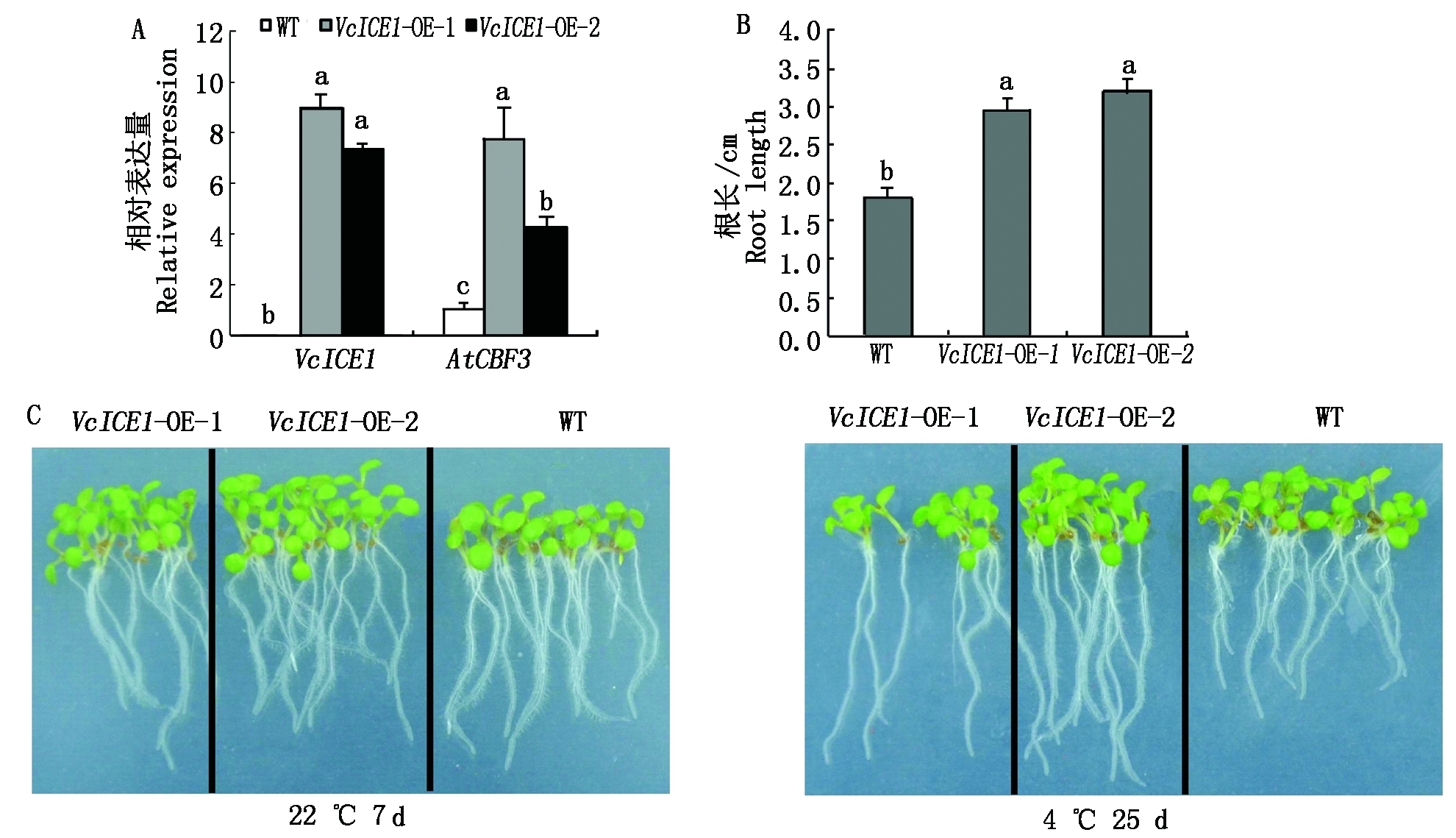
A.定量PCR分析VcICE1、AtCBF3在转基因拟南芥VcICE1-OE-1和VcICE1-OE-2中的表达水平;B.低温处理下野生型和转基因VcICE1拟南芥幼苗的根长;C.野生型和转基因VcICE1拟南芥幼苗耐低温能力分析。
A.qRT-PCR analysis of the expression level of VcICE1, AtCBF3 in VcICE1-OE-1 and VcICE1-OE-2 transgenic Arabidopsis; B.Relative root length of WT,VcICE1-OE-1 and VcICE1-OE-2 transgenic Arabidopsis; C.Phenotypes of WT,VcICE1-OE-1 and VcICE1-OE-2 transgenic Arabidopsis seedlings on chilling tolerance.
图5 VcICE1在野生型拟南芥中过量表达提高抗低温胁迫能力
Fig.5 VcICE1 over-expression increased chilling tolerance in Arabidopsis thaliana

A.烟草叶片瞬时表达试验;B.相对荧光强度。Ⅰ.VcICE1-pGreenⅡ62-SK和AtCBF3pro-LUC共同注射;Ⅱ. pGreenⅡ62-SK空载体和AtCBF3pro-LUC共同注射。
A.Transient expression assay in tobacco leaf;B.Quantitative analysis of relative luminescence.Ⅰ. Co-injection of VcICE1-pGreenⅡ62-SK and AtCBF3pro-LUC;Ⅱ. Co-injection of pGreenⅡ62-SK and AtCBF3pro-LUC.
图6 LUC瞬时表达试验显示VcICE1对AtCBF3表达的影响
Fig.6 Luciferase transient expression assays showing that VcICE1 promotes the expression of AtCBF3

A.相对GUS活性。B.烟草瞬时表达试验:Ⅰ.pRI101空载体和AtCBF3pro-GUS共同注射;Ⅱ.VcICE1-pRI101和AtCBF3pro-GUS共同注射。
A.Quantitative analysis of relative GUS activity; B.Transient expression assay in tobacco leaf:Ⅰ.Co-injection of pRI101 and AtCBF3pro-GUS;Ⅱ.Co-injection of VcICE1-pRI101 and AtCBF3pro-GUS.
图7 GUS瞬时表达试验显示VcICE1对AtCBF3表达的影响
Fig.7 GUS transient expression assays showing that VcICE1 promotes the expression of AtCBF3
目前,在拟南芥中已发现162个bHLH蛋白,其功能涉及抗逆、光信号传导、激素信号传导、生长发育和次生代谢物质合成等多个方面,其作用方式主要通过促进或抑制启动子活性调控靶基因表达[26-35]。本研究从越橘中分离1个VcICE1基因,该基因编码含有bHLH结构域的转录因子。VcICE1可与AtICE1(AtbHLH116)聚为一类,VcICE1与其他植物ICE1蛋白的保守功能域高度相似。因此,VcICE1可能是ICE1在越橘中的同源基因。
组织特异性表达分析显示,拟南芥AtICE1在叶片和茎中表达量较高[15]。油菜BnICE1主要在下胚轴中表达[36]。苹果MdICE1在叶片、休眠芽和花器官中大量表达,在春梢和愈伤组织中表达较弱[37]。在本研究中,VcICE1在幼叶中高表达,在根、枝条和果实中表达相对较低。这种时空表达的差异可能与不同物种特性有关。VcICE1与AtICE1及MdICE1均在叶片中高表达,暗示VcICE1可能与这2个物种的ICE1具有相似的调控植株响应低温的生物学功能。
Feng等 [37]研究表明,苹果MdICE1结合CBF启动子MYC结合位点,在拟南芥、烟草和苹果中超表达MdICE1可显著提高转基因植株的低温抗性[37]。拟南芥AtICE1响应低温诱导表达,可促进其靶基因AtCBF3的转录提高植株低温抗性,沉默AtICE1可抑制AtCBF3的表达,降低植株的低温抗性[38]。本研究发现,越橘VcICE1和VcCBF3均可响应低温信号上调表达,通过转基因试验证明超表达VcICE1可提高拟南芥的低温抗性。瞬时表达试验表明,VcICE1可激活AtCBF3启动子活性。因此,越橘VcICE1与MdICE1及AtICE1的功能和作用方式相似,均是通过CBF途径调控植株对低温信号的响应。
综上所述,本研究克隆获得越橘低温响应因子VcICE1,该基因可响应低温信号而上调表达。VcICE1转基因拟南芥表现提高植株低温抗性的表型。瞬时表达试验发现,VcICE1可促进AtCBF3的表达。该研究结果为深入探讨ICE1基因调节越橘抗低温胁迫机制提供了参考。
[1] 吴林. 中国蓝莓35年—科学研究与产业发展 [J]. 吉林农业大学学报,2016,38(1):1-11.doi:10.13327/j. jjlau.2015.2935.
Wu L. Thirty-five years of research and industry development of blueberry in China [J]. Journal of Jilin Agricultural University,2016,38(1):1-11.
[2] 李亚东,孙海悦,陈丽. 我国蓝莓产业发展报告 [J]. 中国果树,2016,5:1-10.doi:10.16626/j. cnki. Issn1000-8047.2016.05. 001.
Li Y D,Sun H Y,Chen L. Report on the development of blueberry industry in China [J]. China Fruits,2016,5:1-10.
[3] 乌凤章,王贺新,韩慧,郭峰. 防寒措施对越橘越冬微环境和越冬性的影响 [J]. 果树学报,2012,29(2):278-282. doi:10. 13925/j. cnki. gsxb. 2012. 02. 022.
Wu F Z,Wang H X,Han H,Guo F. Effect of winter protection methods on the microenvironment and winter hardiness of blueberry [J]. Journal of Fruit Science,2012,29(2):278-282.
[4] 赵滢,王振兴,许培磊,杨义明,刘迎雪,刘海双,艾军. 山葡萄‘双丰’和‘左优红’叶绿素荧光特性及活性氧代谢与低温伤害的关系 [J]. 园艺学报,2018,45(4):650-658. doi:10. 16420/j. issn. 0513-353x. 2017-0459.
Zhao Y,Wang Z X,Xu P L,Yang Y M,Liu Y X,Liu H S,Ai J. The characteristics of chlorophyll fluorescence and metabolism of reactive oxygen species in relation to the cold injury of Vitis amurensis ′Shuangfeng′ and ′zuoyouhong′ [J]. Acta Horticulturae Sinica,2018,45(4):650 658.
[5] 王海波,程来亮,常源升,孙清荣,陶吉寒,李林光. 苹果矮化砧‘71-3-150’对冷胁迫的生理与转录组响应 [J]. 园艺学报,2016,43(8):1437-1451. doi:10. 16420/j. issn. 0513-353x. 2016-0031.
Wang H B,Cheng L L,Chang Y S,Sun Q R,Tao J G,Li L G. Physiological and transcriptome response of apple dwarfing rootstock to cold stress and cold-resistant genes screening [J]. Acta Horticulturae Sinica,2016,43(8):1437-1451.
[6] Puig C P,Dagar A,Ibanez C M,Singh V,Crisosto C H,Friedman H,Lurie S,Granell A. Pre-symptomatic transcriptome changes during cold storage of chilling sensitive and resistant peach cultivars to elucidate chilling injury mechanism [J]. BMC Genomics,2015,16:245. doi:10. 1186/s12864-015-1395-6.
[7] 单体敏,金鹏,许佳,李晓安,王累,郑永华. 外源甜菜碱处理对冷藏桃果实冷害和品质的影响 [J]. 园艺学报,2015,42(11):2244-2252. doi:10.16420/j.issn.0513-353x.2015-0184.
Shan T M,Jin P,Xu J,Li X A,Wang L,Zheng Y H. Effects of exogenous glycine betaine treatment on chilling injury and quality of cold-stored peach fruits [J]. Acta Horticulturae Sinica,2015,42(11):2244-2252.
[8] 姜寒玉,雷天翔,李唯,何百鋆. 低温胁迫下‘贝达’和‘赤霞珠’葡萄不同组织糖含量及细胞结构的变化 [J]. 果树学报,2015,35(4):604-611.doi:10.13925/j.cnki.gsxb.20140464.
Jiang H Y,Lei T X,Li W,He B Y. Changes of sugar contents in different tissues and cell structure in two grape(Vitis vinifera L.)varieties under low temperature stress [J]. Journal of Fruit Science,2015,35(4):604-611.
[9] 李亚东,卢春雨,裴嘉博. 低温伤害对越橘开花坐果的影响及成因分析 [J]. 中国果树,2008,4:26-28. doi:10. 16626/j.cnki.issn 1000-8047.2008.04.013.
Li Y D,Lu C Y,Pei J B. Effects of low temperature injury on flowering and fruiting of blueberry [J]. 2008,4:26-28.
[10] 魏鑫,魏永祥,刘成,王升,谭永军,杨艳敏. 蓝莓抗寒性研究进展及越冬防寒措施 [J]. 上海农业学报,2015,31(3):147-151. doi:10.15955/j.issn 1000-3924.2015.03.32.
Wei X,Wei Y X,Liu C,Wang S,Tan Y J,Yang Y M. Research progress on cold resistance of blueberry and winter cold proof measure[J].Acta Agriculturae Shanghai,2015,31(3):147-151.
[11] 李亚东,刘海广,唐雪东. 蓝莓栽培图解手册 [M]. 北京:中国农业出版社,2014.
Li Y D,Liu H G,Tang X D. Cultivation graphic manual of blueberry [M]. Beijing:China Agricultural Press,2014.
[12] Thomashow M F. Arabidopsis thaliana as a model for studying mechanisms of plant cold tolerance [M]. New York:Cold Spring Harbor Laboratory Press,1994.
[13] Shinozaki K,Yamaguchi-Shinozaki K. Molecular response to dehydration and low temperature:differences and cross-talk between two stress signaling pathways [J].Current Opinion in Plant Biology,2000,3(3):217-223. doi:10.1016/s1369-5266(00)80068-0.
[14] Thomashow M F. So what′s new in the field of plant clod acclimation Lots! [J]. Plant Physiology,2001,125(1):89-93.doi:10.1104/pp.125.1.89.
[15] Chinnusamy V,Ohta M,Kanrar S,Lee B H,Hong X H,Agarwal M,Zhu J K. ICE1:a regulator of cold-induced transcriptome and freezing tolerance in Arabidopsis [J]. Genes Dev,2003,17:1043-1054.doi:10.1101/gad.1077503.
[16] Liu L Y,Duan L S,Zhang J C,Zhang Z X,Mi G Q,Ren H Z. Cucumber(Cucumis sativus L.)over-expressing cold-induced transcriptome regulator ICE1 exhibits changed morphological characters and enhances chilling tolerance [J]. Scientia Horticulturae,2010,124(1):29-33.doi:10.1016/j.scienta.2009.11.018.
[17] Xiang D J,Hu X Y,Zhang Y,Yin K D. Over-expression of ICE1 gene in transgenic rice improves cold tolerance [J]. Rice Science,2008,15(3):173-178. doi:10. 1016/s1672-6308(08)60039-6.
[18] Budhagatapalli N,Narasimhan R,Rajaraman J,Viswanathan C. Ectopic expression of AtICE1 and OsICE1,transcription factor delays stress-induced senescence and improves tolerance to abiotic stresses in tobacco [J]. Journal of Plant Biochemistry and Biotechnology,2016,25(3):258-293. doi:10.1007/s13562-015-0340-8.
[19] Li J T,Wang L N,Zhu W,Wang N,Xin H P,Li S H. Characterization of two VvICE1 genes isolated from Muscat Hamburg′ grapevine and their effect on the tolerance to abiotic stresses [J]. Scientia Horticulturae,2014,165(165):266-273.doi:10.1016/j.scienta.2013.11.002.
[20] Badawi M,Reddy Y V,Agharbaoui Z,Tominaga Y,Danyluk J,Sarhan F,Houde M. Structure and functional analysis of wheat ICE(Inducer of CBF Expression)genes [J]. Plant and Cell Physiology,2008,49(8):1237-1249. doi:10.1093/pcp/pcn100.
[21] Song Y,Liu H D,Zhou Q,Zhang H J,Zhang Z D,Li Y D,Wang H B,Liu F Z. High-throughput sequencing of highbush blueberry transcriptome and analysis of basic helix-loop-helix transcription factors [J]. Journal of Integrative Agriculture,2017,16(3):591-604. doi:10.1016/s2095-3119(16)61461-2.
[22] Clough S J,Bent A F. Floral dip:a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana [J]. The Plant Journal,1998,16(6):735-743. doi:10.1046/j.1365-313x.1998.00343.X.
[23] Yin X R,Allan A C,Chen K S,Ferguson I B. Kiwifruit EIL and ERF genes involved in regulating fruit ripening [J]. Plant Physiology,2010,153(3):1280-1292. doi:10.1104/pp.110.157081.
[24] Jefferson R A,Kavanagh T A,Bevan M W. GUS fusions:β-glucuronidase as a sensitive and versatile gene fusion marker in higher plants [J]. EMBO Journal,1987,6(13):3901-3907.doi:10.1089/dna.1987.6.583.
[25] An J P,Qu F J,Yao J F,Wang X N,You C X,Wang X F,Hao Y J. The bZIP transcription factor MdHY5 regulates anthocyanin accumulation and nitrate assimilation in apple [J]. Horticulture Research,2017,4:17023. doi:10.1038/hortres.2017.56.
[26] Bailey P C,Martin C,Toledo-Ortiz G,Quail P H,Huq E,Hemi M A,Jakoby M,Werber M,Weisshaar B. Update on the basic helix-loop-helix transcription factor gene family in Arabidopsis thaliana [J]. The Plant Cell,2003,15(11):2497-2502.doi:10.1105/tpc.151140.
[27] Li X L,Zhang H M,Ai Q,Liang G,Yu D Q. Two bHLH transcription factors,bHLH34 and bHLH104,regulate iron homeostasis in Arabidopsis thaliana [J]. Plant Physiology,2017,170:2478-2493. doi:10.1104/pp.15.01827.
[28] Cui J,You C J,Zhu E G,Huang Q,Ma H,Chang F. Feedback regulation of DYT1 by interactions with downstream bHLH factor promotes DYT1 nuclear localization and anther development [J]. The Plant Cell,2016,28:1078-1093. doi:10.1105/tpc.15.00986.
[29] Liang G,Zhang H M,Li X L,Ai Q,Yu D Q. bHLH transcription factor bHLH115 regulates iron homeostasis in Arabidopsis thaliana [J]. Journal of Experimental Botany,2017,68(7):1743-1755. doi:10. 1093/jxb/erx043.
[30] Zhang J,Liu B,Li M S,Feng D R,Jin H L,Wang P,Liu J,Xiong F,Wang J F. The bHLH transcription factor bHLH104 interacts with IAA-LEUCINE RESISTANT3 and modulates iron homeostasis in Arabidopsis [J]. The Plant Cell,2015,27:787-805. doi:10.1105/tpc.114.132704.
[31] Hir R L,Castelain M,Chakraborti D,Moritz T,Dinant S,Bellini C. AtbHLH68 transcription factor contributes to the regulation of ABA homeostasis and drought stress tolerance in Arabidopsis thaliana [J]. Physiologia Plantrum,2017,160:321-327. doi:10.1111/ppl.12549.
[32] Chen X,Huang H,Qi T C,Liu B,Song S S. New perspective of the bHLH-MYB complex in jasmonate-regulated plant fertility in Arabidopsis [J]. Plant Signaling and Behavior,2016,11(2):e1135280. doi:10.1080/15592324.2015.1135280.
[33] Turnbull D,Yang L N,Naqvi S,Breen S,Welsh L,Stephens J,Morris J,Boevink P C,Hedley P E,Zhan J S,Birch P,Gilroy E M. RXLR effector AVR2 up-regulates a brassinosteroid-responsive bHLH transcription factor to suppress immunity [J]. Plant Physiology,2017,174:356-369.doi:10.1104/pp.16.01804.
[34] Bulgakov V,Veremeichik G,Grigorchuk V,Rybin V,Shkryl Y. The rolB gene activates secondary metabolism in Arabidopsis calli via selective activation of genes encoding MYB and bHLH transcription factors [J]. Plant Physiology and Biochemistry,2016,102:70-79. doi:10.1016/j.plaphy.2016.02.015.
[35] Takahashi Y,Ebisu Y,Shimazaki K. Reconstitution of abscisic acid signaling from the receptor to DNA via bHLH transcription factors [J]. Plant Physiology,2017,174:815-822.doi:10.1104/pp.16.01825.
[36] 张腾国,常燕,王娟,王宁,王圆圆,陈琼琼,孙万仓. 油菜BnICE1的克隆及表达分析 [J]. 中国农业科学,2013,46(1):205-214. doi:10.3864/j.issn. 0578-1752.2013.01.024.
Zhang T G,Chang Y,Wang J,Wang N,Wang Y Y,Chen Q Q,Sun W C. Cloning and expression analysis of a BnICE1 from Brassica napus L. [J].Scientia Agricultura Sinica,2013,46(1):205-214.
[37] Feng X M,Zhao Q,Zhao L L,Xie X B,Li H F,Yao Y X,You C X,Hao Y J. The cold-induced basic helix-loop-helix transcription factor gene MdCIbHLH1 encodes an ICE-like protein in apple [J]. BMC Plant Biology,2012,12:22. doi:10.1186/1471-2229-12-22.
[38] Lee B H,Henderson D A,Zhu J K. The Arabidopsis cold-responsive transcriptome and its regulation by ICE1 [J]. The Plant Cell,2005,17(11):3155-3175.doi:10.1105/tpc.105.035568.