“黄金玉米带”使吉林成为中国主要的粮食生产省,在吉林省农业相对发达的中部,秸秆资源产量达到10.87 t/hm2,居全国首位[1-2]。面对大量的秸秆资源,秸秆直接还田是最高效低耗、快捷方便的方式,也是东北地区重点推广技术[3-4]。
秸秆还田不仅能够提高秸秆利用率,而且能够提高土壤有机质与土壤有效养分,是农产品废弃物资源化利用的主要方式[5]。秸秆中含有的微生物会在土壤中分解,增加土壤有益微生物的数量,改善土壤理化性质[6-7]。但是,由于北方低温期较长,秸秆还田后分解较慢,达不到预期的效果。因此,秸秆还田时配施降解菌剂对秸秆进行快速腐解非常有必要。秸秆降解菌剂是能够有效提高秸秆利用率的微生物活体菌剂[8]。实践表明,秸秆降解菌剂的应用对土壤理化性质和酶活性有一定的影响。
土壤酶是动植物和微生物在土壤中活动的产物。它们是极小且极其活跃的土壤成分,对物质转化、能量代谢和修复污染土壤有重要作用[9-11]。土壤酶在土壤中以稳定蛋白质的形式普遍存在,并具有生物催化能力[12-13]。由土壤微生物引起的各种生化过程都是通过它们产生的酶来实现的。土壤酶活性可作为判断土壤生化强度和评价土壤肥力的指标之一[14-15]。
虽然目前很多科研人员都在研究探索适合低温地区秸秆降解的腐熟剂,但是仍然没能完全解决,因此,本研究秸秆降解菌对秸秆降解率及土壤理化性质、土壤酶活性的影响,为秸秆降解菌剂的实际应用提供理论依据。
1 材料和方法
1.1 试验地概况
试验地位于吉林农业大学培养试验田(123°20′45″ E,43°47′42″ N),年均降雨量为549.6 mm,年均蒸发量为1 490 mm,年积温2 750 ℃,年平均气温为4.9 ℃,平均最高气温29.3 ℃,最低气温-20.4 ℃,无霜期140 d[16]。供试土壤为草甸黑土,土壤pH 值为5.95,有机质含量为22.65 g/kg,碱解氮含量103.25 mg/kg,速效磷、速效钾含量分别为24.46 mg/kg、103.27 mg/kg,铵态氮、硝态氮含量分别为25.05,23.87 mg/kg。
1.2 试验设计
玉米秸秆和土壤均采自吉林农业大学试验田。试验设置5个处理: S(秸秆+土); LB(低温菌剂+秸秆+土); NB(常温菌剂+秸秆+土); MB(低温菌剂+常温菌剂+秸秆+土)(注:以下将低温菌剂+常温菌剂称为混合菌剂);另外不加菌剂和秸秆的原土作为对照CK。每个处理3次重复。
每个尼龙小袋装入1 kg黑土、4 g玉米秸秆和0.04 g菌剂,充分混匀。然后将尼龙小袋埋入地下,翻埋深度为3~5 cm。试验时间为8-11月,共为期100 d。
1.3 土样采集
分别在15,30,50,70,100 d时取样,每次每个处理取一个尼龙小袋,共取5次,分别测定尼龙小袋中的土壤理化性质、土壤酶活性以及秸秆降解率。
1.4 试验方法
秸秆降解率:将混入尼龙小袋中的秸秆挑拣出来,冲洗干净,然后80 ℃烘干至恒质量。秸秆降解率=(培养前秸秆质量-培养n d时秸秆质量)/培养前秸秆质量×100%。
采用硫代硫酸钠滴定法测定土壤转化酶活性,以24 h后1 g土壤消耗0.1 N硫代硫酸钠的毫升数表示;采用靛酚比色法测定土壤脲酶活性,以24 h后1 g土壤中NH3-N的毫克数表示;采用高锰酸钾滴定法测定土壤过氧化氢酶活性,以1 g土壤消耗0.1 mol/L高锰酸钾的毫升数表示;采用磷酸苯二钠比色法测定土壤酸性磷酸酶活性,以24 h后1 g土壤中酚的微克数表示;采用3,5-二硝基水杨酸比色法测定土壤纤维素酶活性,以72 h后1 g干土生成葡萄糖的毫克数表示[17-18]。
土壤理化性质采用常规土壤农化分析方法测定[19-20]。
1.5 数据处理
数据均采用Excel 2016和SPSS 24.0进行分析。
2 结果与分析
2.1 不同菌剂对玉米秸秆降解效果的影响
从图1可知,处理LB和MB的秸秆降解率与处理S相比,有显著性差异(P<0.05);而处理NB的秸秆降解速度较缓慢,与处理S差异性较小,但是整体都是呈上升趋势。从培养天数上看,处理LB在培养初期,秸秆降解率并没有明显的变化趋势,而在培养30 d后,降解率明显提高;处理NB和MB的秸秆降解率则随着培养天数的增加稳定提高。各个处理均在培养100 d时,秸秆降解率达到最高,具体表现为:MB(86%)>LB(81%)>NB(77%)>S(71%)。
2.2 不同菌剂处理对土壤理化性质的影响
由表1可知,随培养天数的增加,土壤中各养分含量均显著提高(P<0.05),100 d达到最大值;同一培养阶段,不同处理间的土壤速效磷、速效钾、有机质、碱解氮、铵态氮、硝态氮含量和pH值均差异性显著,表现为:MB>LB>NB>S>CK。
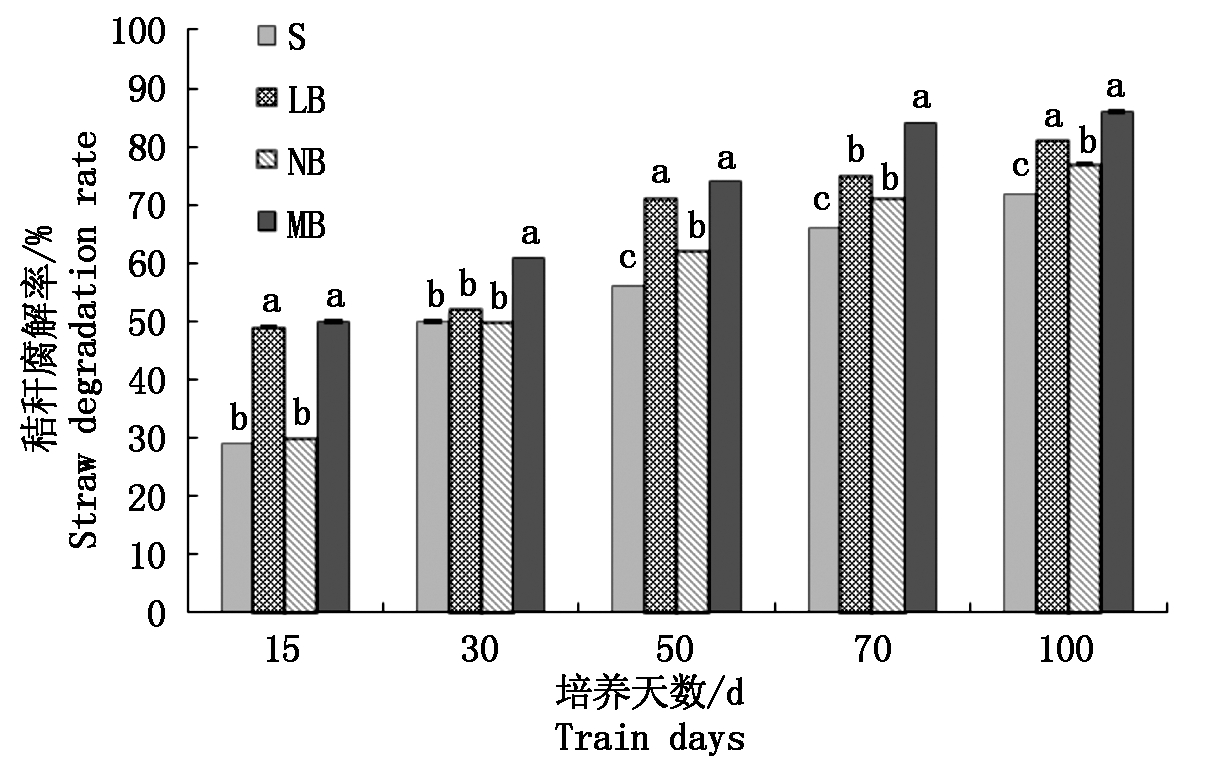
图中a、b、c表示处理间差异显著。
In the figure, a, b, and c represent significant
differences between treatments.
图1 玉米秸秆降解率的变化
Fig.1 The degradation rate of the corn stover
表1 不同菌剂处理对土壤理化性质的影响
Tab.1 Effects of different fungicides on soil physical and chemical properties

处理TreatmentspH值pH value有机质/(g/kg)Organic matter碱解氮/(mg/kg)Available N速效磷/(mg/kg)Available P速效钾/(mg/kg)Available K铵态氮/(mg/kg)Ammonium nitrogen硝态氮/(mg/kg)Nitrate nitrogen15 dCK5.95±0.04b22.65±1.39b103.25±1.13b24.46±0.53a103.27±0.16c25.05±0.26b23.87±11.29aS6.00±0.03b18.28±1.99a102.78±2.83b15.80±1.59b132.40±0.22d12.03±2.28b16.37±14.74bLB6.42±0.13a20.06±2.73a113.87±3.51a19.10±0.42ab134.48±0.51b16.34±3.45ab20.05±20.84aNB6.39±0.02a19.46±1.96a106.05±1.60b17.47±1.67ab133.52±0.26c13.36±1.56b17.51±14.59bMB6.45±0.01a20.96±2.51a114.45±4.81a19.80±0.57a151.74±0.43a18.21±7.54a21.94±9.73ab30 dCK6.07±0.03b22.73±1.36a103.37±1.13b24.51±1.28b106.45±0.21a25.13±1.24a23.91±4.59bS6.31±0.05b19.45±0.79d105.47±0.73c18.47±2.25a132.97±0.79c14.07±1.15a24.07±9.89aLB6.37±0.03a23.31±1.37b111.53±1.07b19.73±6.36a134.50±0.31b16.24±1.67a26.32±5.27aNB6.32±0.05a20.74±1.80c107.80±1.53bc19.60±2.31a133.48±0.40c14.43±4.58a24.43±4.69aMB6.53±0.15a24.27±0.54a118.37±3.78a20.40±5.38a135.54±0.27a19.73±14.45a26.24±12.24a50 dCK6.13±0.01b22.81±2.17a105.12±0.83b24.62±1.52a108.36±0.19a25.25±0.72b24.07±0.52aS6.34±0.02b22.98±1.80b109.67±5.78a22.13±1.81a134.00±0.38a15.51±0.65b21.33±0.65bLB6.45±0.12a23.74±0.79b116.55±1.60a25.80±5.29a135.71±0.40a20.54±1.61b24.56±31.55aNB6.42±0.03a23.47±0.54a113.87±2.58a22.15±0.49a135.30±0.60a19.41±0.94b22.70±0.50bMB7.14±0.04a24.58±1.37b117.95±5.50a28.70±5.80a139.80±5.97a27.79±6.49a26.25±18.19b70 dCK6.21±0.013b23.06±1.06a107.37±1.28a24.74±1.61a112.23±0.29b25.31±1.76b24.24±4.25aS6.24±0.15b23.63±0.24a113.52±2.25a24.13±6.63a134.28±0.06c24.11±4.49a24.43±30.49aLB6.52±0.03ab24.58±1.14a120.63±19.59a24.75±0.51b135.56±0.25b26.02±6.49a27.29±2.45bNB6.41±0.06b24.25±0.55a114.33±1.07a24.27±2.39b134.65±0.39c25.54±0.91a25.26±0.42bMB6.71±0.25a24.67±0.72a121.63±44.66a28.00±4.16b136.67±0.26a30.74±0.52a28.19±0.32b100 dCK6.28±0.05b23.28±1.12a109.57±1.03b24.81±1.27a121.45±0.34b26.14±2.16a25.02±1.42aS6.35±0.02b24.98±1.94a113.87±3.84b24.40±3.94a134.73±0.22c31.47±2.11c28.23±0.30cLB6.35±0.06b26.27±0.77a116.67±2.25b29.73±3.00a135.64±0.31b36.23±15.80a34.15±1.01bNB6.41±0.04a25.87±1.06a114.80±3.05b24.80±3.10a135.38±0.35b36.22±0.89bc32.81±7.57aMB6.58±0.05a26.70±0.31a122.03±1.07a31.10±1.56a136.31±0.29a38.20±0.32b34.52±10.55ab
注:表中数据为平均值±标准差,同列数据后不同小写字母表示差异显著(P<0.05)。
Note: The data in the table were ![]() different lowercase letters in the same colum indicated significant difference (P<0.05).
different lowercase letters in the same colum indicated significant difference (P<0.05).
2.3 不同菌剂处理对土壤酶的影响
2.3.1 不同菌剂处理下土壤脲酶活性的变化 从图2可以看出,处理NB在整个培养过程中,随着培养天数的增加,土壤脲酶活性增长缓慢,差异不显著;处理LB的土壤脲酶活性在培养末期达到了8.76 mg/(g·d),较培养初期高出32.13%,同时高于无菌对照87.05%。在培养过程中,发现处理MB的增长幅度最大,培养末期较培养初期增长了1.7倍;同时处理LB和NB的土壤脲酶活性也较培养初期增长了1.2,1.3倍,而无菌处理S的土壤脲酶活性变化不明显。
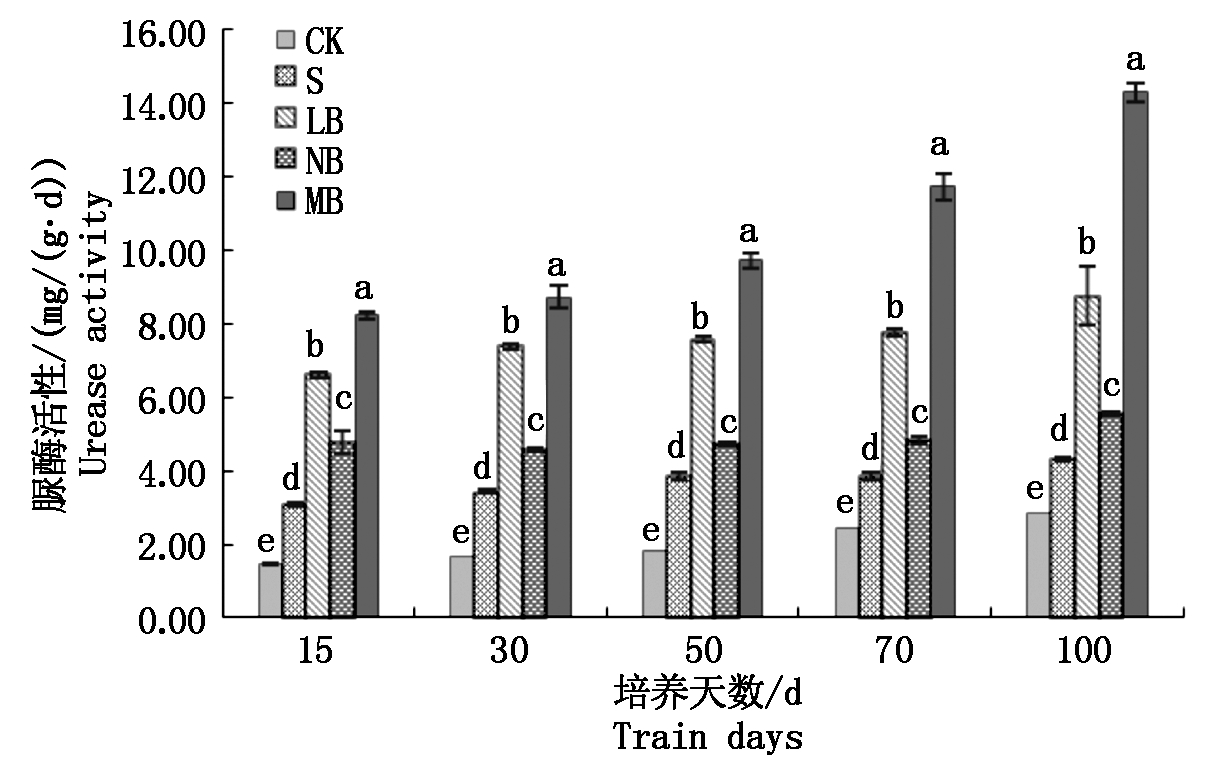
图2 土壤脲酶活性的变化
Fig.2 Changes in soil urease activity
2.3.2 不同菌剂处理下土壤转化酶活性的变化 从图3可以看出,各个处理土壤转化酶的活性随着培养时间的增加而提高,差异性较小。处理LB的土壤转化酶的活性在培养过程中由9.82 mL/(g·d)增加到10.49 mL/(g·d),增加了6.8%;处理NB的土壤转化酶的活性由9.80 mL/(g· d)增加到10.23 mL/(g·d),增加了4.4%,2个处理的土壤转化酶活性上升幅度都较小,差异不显著;而处理MB的土壤转化酶活性由9.95 mL/(g·d)增加到11.70 mL/(g·d),提高了17.6%,提高幅度相对最大,且明显高于无菌对照S和空白对照CK。
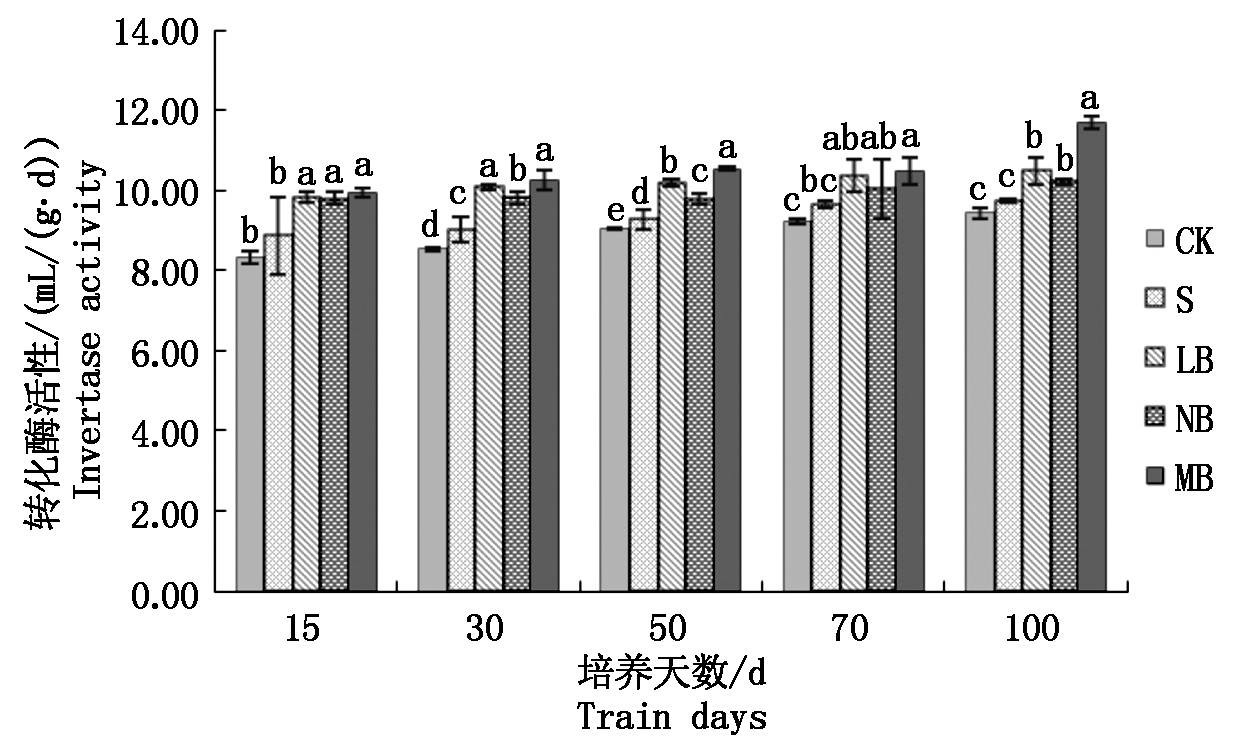
图3 土壤转化酶活性的变化
Fig.3 Changes of soil invertase activity
2.3.3 不同菌剂处理下土壤酸性磷酸酶活性的变化 从图4可以看出,处理NB的土壤酸性磷酸酶活性在每一个培养阶段都略高于处理S,差异不显著,但总体稳定提高,最高值出现在培养100 d时,活性为0.51 μg/(g·d);处理LB的土壤酸性磷酸酶活性在每一个培养阶段都明显高于处理S,但在培养过程中,随着培养天数的增加,处理LB的土壤酸性磷酸酶活性上升缓慢,培养末期仅高于初期0.25 μg/(g·d);而处理MB的土壤酸性磷酸酶活性在培养末期显著高于其他处理,分别高于处理NB、LB、S和CK 27.45%,12.07%,47.73%,85.71%。
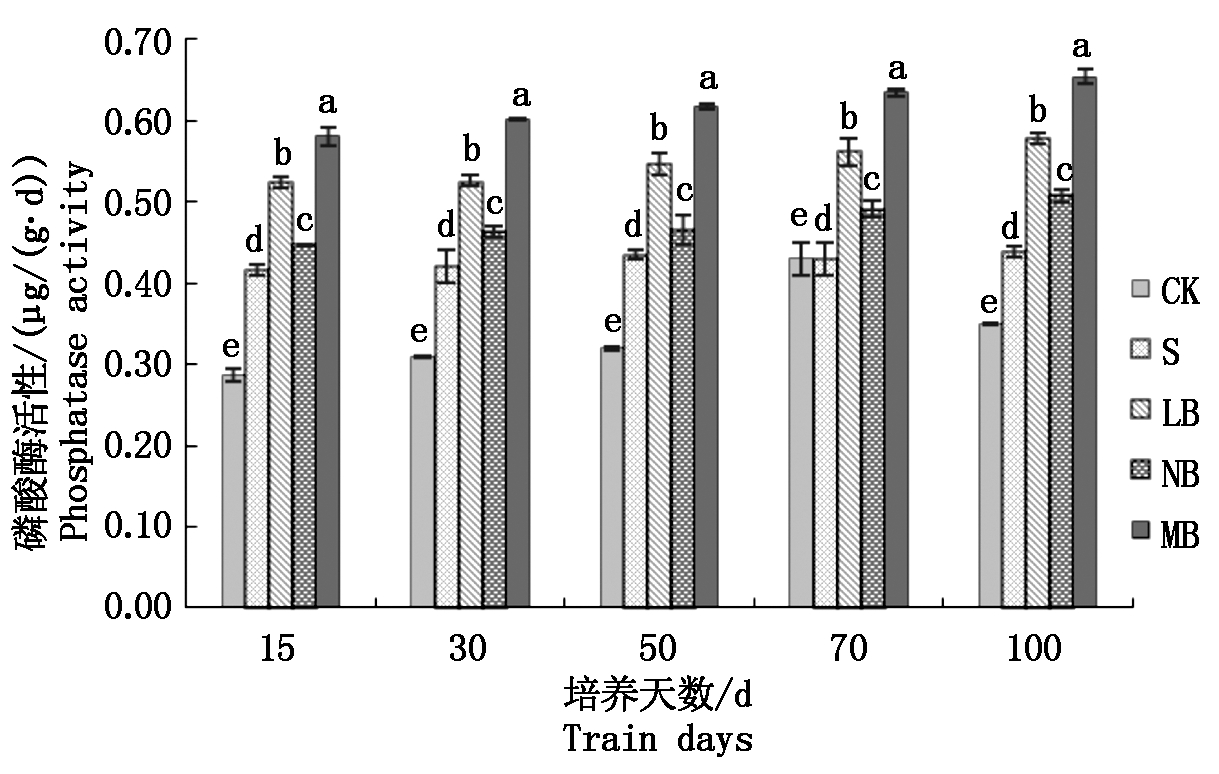
图4 土壤磷酸酶活性的变化
Fig.4 Changes of soil phosphatase activity
2.3.4 不同菌剂处理下土壤过氧化氢酶活性的变化 由图5可知,在培养初期,处理LB的过氧化氢酶活性变化不明显,只是保持平稳,在培养50 d 后过氧化氢酶活性开始显著提高,增加到5.78 mL/(g·d),较培养初期提高了19.67%;处理NB的土壤过氧化氢酶活性随着培养天数的增加由4.34 mL/(g·d) 增加到7.69 mL/(g·d),同时较处理S高出11.45%;处理MB的土壤过氧化氢酶活性由5.28 mL/(g·d)增加到8.36 mL/(g·d),增加了58.33%,差异性显著;在培养末期,各个处理的土壤转化酶活性表现为MB>LB>NB>S>CK。
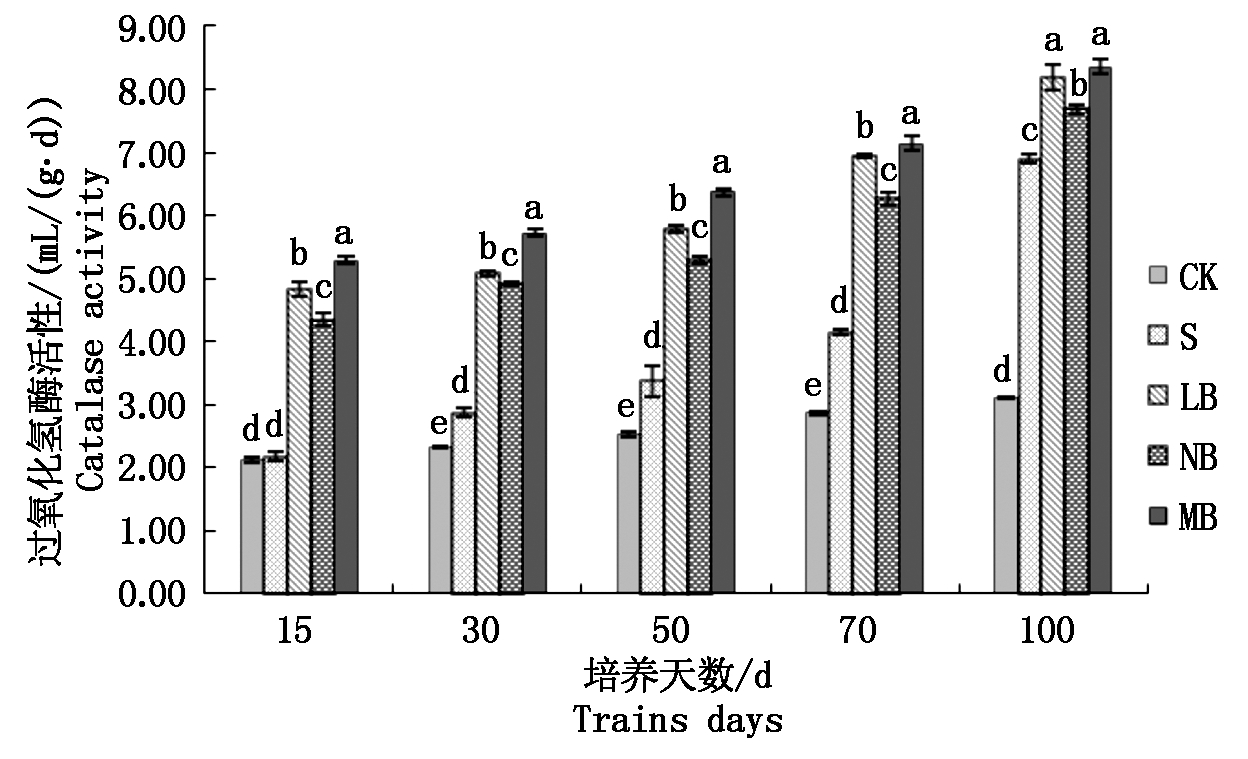
图5 土壤过氧化氢酶活性的变化
Fig.5 Changes of soil catalase activity
2.3.5 不同菌剂处理下土壤纤维素酶活性的变化 从图6可以看出,处理LB的土壤纤维素酶活性在培养初期略高于处理S,差异不显著,但在培养50 d后开始显著提高,最高值出现在培养100 d时,活性为0.30 mg/(g·d);处理NB的土壤纤维素酶活性随着培养天数的增加缓慢提高,培养末期高于初期0.02 mg/(g·d),同时分别高于CK和处理S 0.04,0.02 mg/(g·d);处理MB的土壤纤维素酶活性随着培养天数的增加,呈现显著提高的趋势,100 d时活性达到0.35 mg/(g·d)。
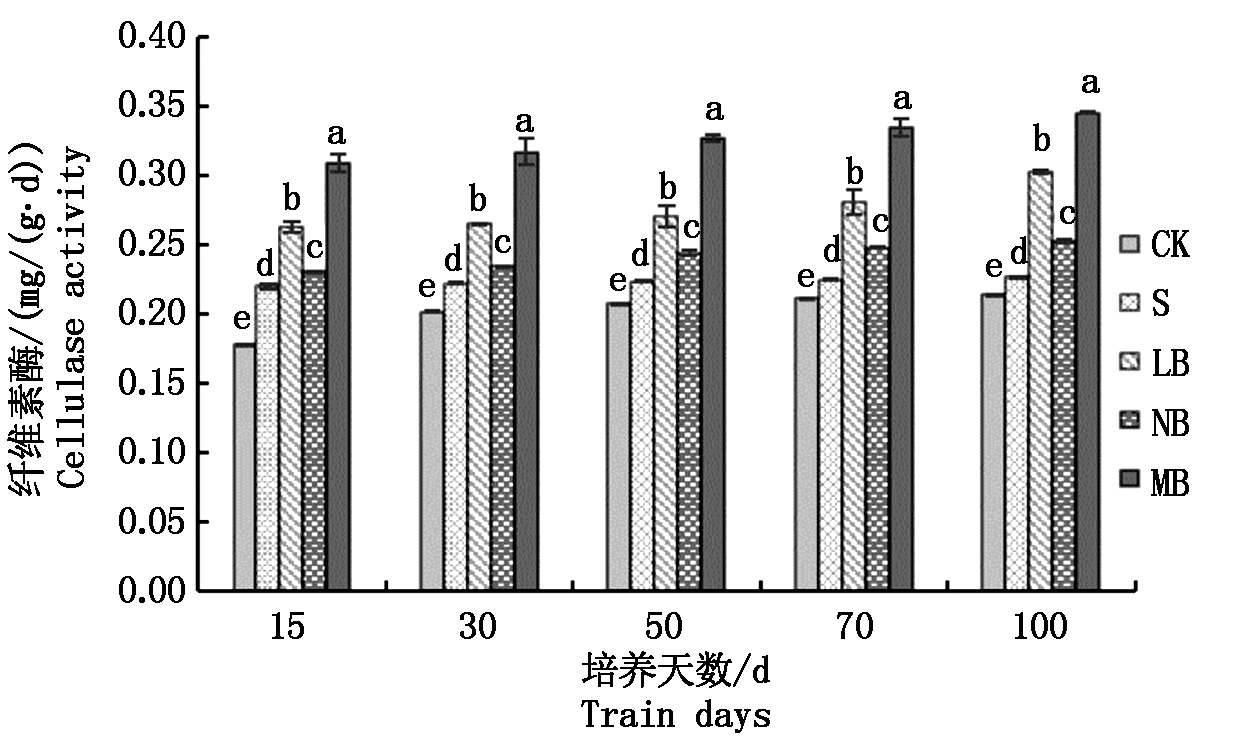
图6 土壤纤维素酶活性的变化
Fig.6 Changes of soil cellulase activity
2.4 土壤理化性质与土壤酶的关系
由表2可知,土壤过氧化氢酶活性与硝态氮、碱解氮含量呈极显著正相关,与其他理化性质无相关性;土壤磷酸酶活性与硝态氮、碱解氮、速效钾、速效磷含量呈显著正相关,而与土壤pH值和铵态氮含量没有相关性;土壤脲酶活性与铵态氮含量呈极显著正相关,与速效钾、速效磷含量和pH值呈显著正相关,而与硝态氮和碱解氮含量无相关性;土壤转化酶活性与土壤铵态氮、速效钾含量和pH值呈显著正相关,而与其他理化性质无相关性;土壤纤维素酶活性与硝态氮、铵态氮、速效钾、速效磷含量呈显著正相关,与碱解氮含量和pH值无相关性。
表2 土壤理化性质与土壤酶之间的相关性分析
Tab.2 Correlation analysis between soil physical and chemical properties and soil enzymes
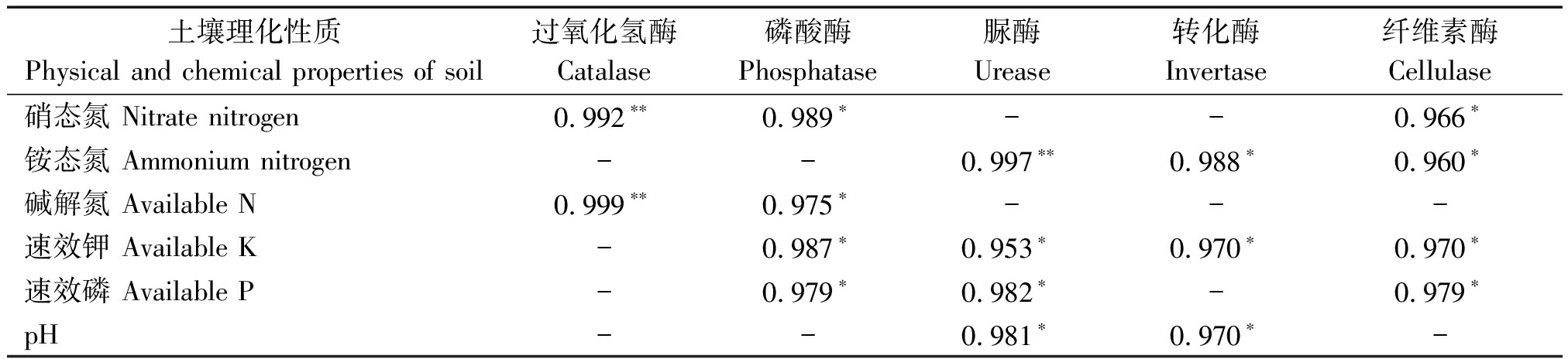
土壤理化性质Physical and chemical properties of soil过氧化氢酶Catalase磷酸酶Phosphatase脲酶Urease转化酶Invertase纤维素酶Cellulase硝态氮 Nitrate nitrogen0.992∗∗0.989∗--0.966∗铵态氮 Ammonium nitrogen--0.997∗∗0.988∗0.960∗碱解氮 Available N0.999∗∗0.975∗---速效钾 Available K-0.987∗0.953∗0.970∗0.970∗速效磷 Available P-0.979∗0.982∗-0.979∗pH--0.981∗0.970∗-
注:*.差异达到显著(P<0.05);**.差异达到极显著(P<0.01)。
Note: *.Significant difference (P<0.05);**. Extremely significant difference (P<0.01).
3 讨论与结论
本研究发现,秸秆降解菌剂配合秸秆还田到土壤中显著影响土壤理化性质,且随着培养时间的延长,土壤的理化性质变化显著。有机质、速效磷和速效钾等土壤理化性质的变化,可能是因为秸秆中本身富含有机质和养分,添加菌剂加快了秸秆的降解,从而促进了有机质和养分的释放,使土壤获得了更多的有效养分[21]。有很多研究表明,秸秆还田配施秸秆降解菌剂能够提高土壤理化性质[22-23],慕兰等[24]的研究结果表明,经过150 d的腐解,有机质等各养分含量均呈现不同的增加趋势。本研究中秸秆还田配施菌剂使土壤pH值从5.95提高至6.58,土壤碱解氮含量从103.25 mg/kg增加到122.03 mg/kg,土壤速效磷、速效钾、有机质等含量也均有不同程度的提高,这与上述研究结果一致。
李鹤[25]和青格尔等[26]的研究结果表明,与CK相比,添加降解菌剂处理后的秸秆降解率较高,表现出较好的促分解效果。本研究中,经过100 d的培养,添加低温菌剂、常温菌剂和混合菌剂处理的秸秆降解率分别为81%,77%,86%,而CK为72%,说明添加降解菌剂可有效促进秸秆的分解速率。同时研究表明,施加混合菌剂处理的分解效果优于施加低温菌剂处理和常温菌剂处理,这与上述研究结果一致。这可能是因为秸秆降解菌剂中含有大量的霉菌、细菌、酵母菌和芽孢杆菌等,秸秆还田后这些菌大量繁殖,能够有效促进作物秸秆分解[27]。
酶作为土壤中最活跃的体系,与土壤肥力关系密切,是评价土壤肥力的重要指标[28]。本研究表明,添加低温菌剂对土壤酶活性的作用比常温菌剂显著,其中效果最为明显是脲酶,在培养100 d时,施用低温菌剂的土壤脲酶活力为8.76 mg/(g·d),高于常温菌剂处理57.27%,同时研究发现,2种菌剂同时施用的混合菌剂对提高土壤酶活性的效果最佳,施用混合菌剂处理的脲酶活性在培养末期达到14.30 mg/(g·d),明显高于其他处理;本研究中,土壤过氧化氢酶、纤维素酶、转化酶和酸性磷酸酶活性提高幅度最大的均为混合菌剂处理。钱海燕等[29]的研究也表明,秸秆还田与微生物菌剂的结合可以促进土壤酶活性的提高,同时混合菌剂效果最好。本研究结果与之相一致。分析产生上述结果的原因,可能是因为添加混合菌剂,能够有效促进秸秆的腐殖化速度以及有效元素的积累,同时提高了土壤有机碳,更有利于微生物的活动与土壤酶活性的提高[30]。
本试验研究表明:与对照相比,秸秆配施降解菌剂有利于玉米秸秆的降解以及土壤速效养分和酶活性的增加。尤其是混合菌剂(低温菌剂+常温菌剂)处理,对秸秆降解效果以及土壤理化性质和土壤酶活性的影响优于低温菌剂处理和常温菌剂处理。由此可知,秸秆降解菌剂能够有效地分解作物秸秆,秸秆经菌剂发酵后,协调了土壤酸碱度和土壤氮、磷、钾养分的供应,提高土壤的保水保肥能力。
[1] 任林举.吉林“黄金玉米带”是荣耀的王冠,还是沉重的翅膀?[J].黑龙江粮食, 2017(6):30-33. doi:10.3969/j.issn.1671-6019.2017.06.014.
Ren L J. Jilin "golden corn belt" is the glory of the crown, or heavy wings?[J].Journal of Heilongjiang Grain, 2017(6):30-33.
[2] Yang Y L, Zhang P D, Zhang W L, Tian Y S, Zheng Y H, Wang L S. Quantitative appraisal and potential analysis for primary biomass resources for energy utilization in China[J].Renewable and Sustainable Energy Reviews, 2010, 14(9):3050-3058. doi:10.1016/j.rser.2010.07.054.
[3] 王金武,唐汉,王金峰.东北地区作物秸秆资源综合利用现状与发展分析[J].农业机械学报,2017,48(5):1-21. doi:10.6041/j.issn.1000-1298.2017.05.001.
Wang J W, Tang H, Wang J F. Comprehensive utilization status and development analysis of crop straw resource in Northeast China[J]. Journal of Agricultural Machinery, 2017, 48(5):1-21.
[4] 余坤,冯浩,李正鹏,王增丽.秸秆还田对农田土壤水分与冬小麦耗水特征的影响[J].农业机械学报,2014,45(10):116-123. doi:10.6041/j.issn.1000-1298.2014.10.019.
Yu K, Feng H, Li Z P, Wang Z L. Effects of different pretreated straw on soil water content and water consumption characteristics of winter wheat[J]. Journal of Agricultural Machinery, 2014, 45(10):116-123.
[5] 杨会萍. 秸秆还田技术研究及应用进展[J]. 农业开发与装备, 2017(10):61. doi: 10.3969/j.issn.1673-9205.2017.10.052.
Yang H P. Research and application progress of straw mulching technology[J]. Agricultural Development and Equipment, 2017(10):61.
[6] Silva A P D, Babujia L C, Franchini J C, Ralisch R, Hungria M, Guimarães M D F. Soil structure and its influence on microbial biomass in different soil and crop management systems[J]. Soil and Tillage Research, 2014, 142:42-53. doi: 10.1016/j.still.2014.04.006.
[7] 孙婧,田永强,高丽红,彭杏敏,佟二建.秸秆生物反应堆与菌肥对温室番茄土壤微环境的影响[J].农业工程学报, 2014(6):153-164. doi: 10.3969/j.issn.1002-6819.2014.06.019.
Sun J, Tian Y Q, Gao L H, Peng X M, Tong E J. Effects of straw biological reactor and microbial agents on physicochemical properties and microbial diversity of tomato soil in solar greenhouse[J]. Transactions of the Chinese Society of Agricultural Engineering, 2014(6):153-164.
[8] 宋志伟,陈露露,潘宇,钟子楠,王晶.3种菌剂对水稻秸秆降解性能的影响[J].生态环境学报,2018, 27(11):158-165. doi: 10.16258/j.cnki.1674-5906.2018.11.021.
Song Z W, Chen L L, Pan Y, Zhong Z N, Wang J. Effects of three bacteriological agents on degradation of rice straw[J]. Ecology and Environmental Sciences, 2018, 27(11):158-165.
[9] 高大响, 黄小忠, 王亚萍. 秸秆还田及腐熟剂对土壤微生物特性和酶活性的影响[J]. 江苏农业科学, 2016,44(12):468-471. doi: 10.15889/j.issn.1002-1302.2016.12.139.
Gao D X, Huang X Z, Wang Y P. Effects of straw mulching and curing agents on soil microbial characteristics and enzyme activity[J]. Jiangsu Agricultural Science, 2016, 44(12):468-471.
[10] Saritha M, Arora A, Lata. Biological pretreatment of lignocellulosic substrates for enhanced delignification and enzymatic digestibility[J]. Indian Journal of Microbiology, 2012, 52(2):122-130. doi: 10.1007/s12088-011-0199-x.
[11] 赵伟,潘延欣,靳雯然,刘畅,王宏燕,刘保平,刘佳斌,李传宝.低温菌剂降解秸秆还田对东北黑土微生物活性的影响[J].湖北农业科学, 2014, 53(17): 4020-4024. doi: 10.3969/j.issn.0439-8114.2014.17.010.
Zhao W, Pan Y X, Jin W R, Liu C, Wang H Y, Liu B P, Liu J B, Li C B. Effects of Low-temperature agent degrading straw and returning to field on microbial activity in black soil[J]. Hubei Agricultural Sciences, 2014, 53(17): 4020-4024.
[12] 崔振波.茶树生长中土壤微生物与土壤酶的相关性研究[J].福建茶叶,2016,38(12):207-208. doi: 10.3969/j.issn.1005-2291.2016.12.136.
Cui Z B. Study on the correlation between soil microorganism and soil enzyme in the growth of tea tree[J]. Fujian Tea, 2016, 38(12):207-208.
[13] Hosseini S S, Lakzian A, Halajnia A. The effect of EDTA and citric acid on soil enzymes activity, substrate induced respiration and pb availability in a contaminated soil (In Persian with English extended abstract)[J].Majallah-i āb va Khāk, 2017, 30(6):2032-2045. doi: 10.22067/jsw.v30i6.50232.
[14] 边雪廉, 岳中辉, 焦浩, 王惠一, 隋海霞, 赵文磊. 土壤酶对土壤环境质量指示作用的研究进展[J]. 土壤, 2015, 47(4):634-640. doi: 10.13758/j.cnki.tr.2015.04.002.
Bian X L, Yue Z H, Jiao H, Wang H Y, Sui H X, Zhao W L. Research progress of soil enzyme on soil environmental quality indicator[J]. Soil, 2015, 47(4):634-640.
[15] Kavkler K, Gunde-Cimerman N, Zalarš P, Demšard. Fungal contamination of textile objects preserved in Slovene museums and religious institutions[J].International Biodeterioration & Biodegradation,2015, 97:51-59. doi: 10.1016/j.ibiod.2014.09.020.
[16] 武俊男,刘昱辛,王天野,高强,吴海燕,刘淑霞.长期施肥对黑土玉米田钾素Q/I特性的影响[J].华北农学报,2018, 33(5):185-187. doi: 10.7668/hbnxb.2018.05.025.
Wu J N, Liu Y X, Wang T Y, Gao Q, Wu H Y, Liu S X. Effect of long-term fertilization on potassium Q/I characteristics in black soil maize field[J]. North China Agricultural Journal, 2018, 33(5):185-187.
[17] 关松荫.土壤酶及其研究法[M].北京:农业出版社,1986.
Guan S Y. Soil enzyme and its research method[M].Beijing: Agricultural Press, 1986.
[18] 林先贵.土壤微生物研究原理与方法[M].北京:高等教育出版社,2010.
Lin X G. Principles and methods of soil microbial research[M]. Beijing: Higher Education Press, 2010.
[19] 鲍士旦.土壤农化分析[M]. 3版.北京:中国农业出版社, 2000.
Bao S D. Soil agrochemical analysis[M]. 3rd ed. Beijing:China Agricultural Press, 2000.
[20] 中国土壤学会.土壤农业化学常规分析方法[M].北京:科学出版社,1983.
Soil Science Society of China.Conventional analytical methods for soil agricultural chemistry[M].Beijing:Science Press,1983.
[21] 盛海君,施凯峰,牛东,杨静,朱新开,马爱军.秸秆添加快腐菌剂还田对麦季土壤养分和小麦产量与品质的影响[J].江苏农业科学,2017,45(19):174-177. doi: 10.15889/j.issn.1002-1302.2017.19.037.
Sheng H J, Shi K F, Niu D, Yang J, Zhu X K, Ma A J. Effects of straw mulching on soil nutrients in wheat season and wheat yield and quality[J]. Jiangsu Agricultural Science, 2017, 45(19):174-177.
[22] 李文斌, 张建忠, 莫丽萍. 有机物料腐熟剂在玉米秸秆还田中的应用效果研究[J]. 河南农业, 2015(17):19-20. doi: 10.3969/j.issn.1006-950X.2015.17.015.
Li W B, Zhang J Z, Mo L P. Study on the application effect of organic material decaying agent in returning corn stalk to field[J]. Henan Agriculture, 2015(17):19-20.
[23] 邹清祺,郝起礼,陈田庆.不同秸秆还田方式对土壤有机质及速效养分的影响[J].西部大开发(土地开发工程研究),2017,2(7):62-67.
Zou Q Q, Hao Q L, Chen T Q. Effects of different models of wheat straw returning on organic matter accumulation and available nutrient status of soil[J]. Western Development (Land Development Engineering Research), 2017,2(7):62-67.
[24] 慕兰,孙笑梅,王立河,王喜枝,姚利娟.不同秸秆腐熟剂在玉米秸秆粉碎还田中的应用效果研究[J].现代农业科技,2013(16):215-216. doi: 10.3969/j.issn.1007-5739.2013.16.139.
Mu L, Sun X M, Wang L H, Wang X Z, Yao L J. Research on the application effect of different straw ripening agents in comminuted corn straw returning to field[J]. Modern Agricultural Technology, 2013(16):215-216.
[25] 李鹤.低温秸秆降解菌的酶活、降解效果及对土壤养分、酶活的影响[D].长春:吉林农业大学, 2015.
Li H. Enzyme activity and degradation effect of low-temperature straw degradation bacteria on soil nutrients and enzyme activity[D]. Changchun:Jilin Agricultural University, 2015.
[26] 青格尔,高聚林,于晓芳,王振,王志刚,闹干朝鲁,胡树平,孙继颖.玉米秸秆低温高效降解复合菌系GF-20的温度和pH适应性研究[J].西北农林科技大学学报(自然科学版),2017,45(1):156-164. doi: 10.13207/j.cnki.jnwafu.2017.01.022.
Qing G E, Gao J L, Yu X F, Wang Z, Wang Z G, Nao G C L, Hu S P, Sun J Y. Study on temperature and pH adaptability of low temperature and high efficiency degradation composite strain GF-20 of corn stover[J]. Journal of Northwest Agricultural and Forestry University (Natural Science Edition), 2017, 45(1):156-164.
[27] 胡海红,孙继颖,高聚林,王振,包闹干朝鲁,胡树平,青格尔.低温高效降解玉米秸秆复合菌系发酵条件优化及腐解菌剂的研究[J].农业环境科学学报,2016,35(8):1602-1609. doi: 10.11654/jaes.2016-0110.
Hu H H, Sun J Y, Gao J L, Wang Z, Bao N G C L, Hu S P, Qing G E. Study on optimization of fermentation conditions and fungicides for degrading corn stover at low temperature and high efficiency[J]. Journal of Agricultural Environmental Science, 2016, 35(8):1602-1609.
[28] 张英洁,靳英华,谷晓楠,许嘉巍,陶岩,贺红士,王嫒林,刘羽霞,牛莉平.长白山苔原带植被变化与土壤微生物、酶活性及土壤肥力的相关性[J].生态学杂志, 2017,36(11):94-101. doi:10.13292/j.1000-4890.201711.014.
Zhang Y J, Jin Y H, Gu X N, Xu J W, Tao Y, He H S, Wang Y L, Liu Y X, Niu L P. Correlation of vegetation change and soil microorganism, enzyme activity and soil fertility in tundra of changbai mountain[J]. Journal of Ecology, 2017, 36(11):94-101.
[29] 钱海燕,杨滨娟,黄国勤,严玉平,樊哲文,方豫.秸秆还田配施化肥及微生物菌剂对水田土壤酶活性和微生物数量的影响[J].生态环境学报,2012,21(3):440-445. doi: 10.3969/j.issn.1674-5906.2012.03.008.
Qian H Y, Yang B J, Huang G Q, Yan Y P, Fan Z W, Fang Y. Effects of returning rice straw to fields with fertilizers and microorganism liquids on soil enzyme activities and microorganisms in paddy fields[J]. Journal of Ecological Environment, 2012, 21(3):440-445.
[30] 潘延欣.秸秆还田配施低温菌剂对黑土氮碳及细菌多样性的影响[D].哈尔滨:东北农业大学,2015. doi: 10.7666/d.Y2771820.
Pan Y X. Effects of low temperature bacteria on nitrogen and carbon and bacterial diversity in black soil[D].Harbin:Northeast Agricultural University,2015.