胡萝卜软腐果胶杆菌(Pectobacterium carotovorum(Pc))是自然条件下普遍存在、寄主范围和地理分布最为广泛的植物病原菌之一[1]。Pc表现出高度的遗传多样性,可被进一步划分为3个亚种:胡萝卜软腐果胶杆菌胡萝卜亚种P.carotovorum subsp. carotovorum(Pcc)、胡萝卜软腐果胶杆菌黄檀亚种P. carotovorum subsp. odoriferum(Pco)和胡萝卜软腐果胶杆菌巴西亚种P. carotovorum subsp. brasiliensis(Pcb)[2]。不同于以往认为的引发白菜细菌性软腐病的病原菌为Pcc亚种[3-4],对北京地区白菜型油菜(Brassica rapa subsp. chinensis)软腐病株进行分离,得到了Pcb菌株[5-6],而引发该地区芹菜(Apium graveolens)软腐病原菌则以Pco为主[7-8];可见,Pc不同亚种菌株具有各自的寄主偏好性。快菜(苗用大白菜,Brassica rapa subsp. pekinensis)因具有口感好、营养价值高和生长周期短等优点,已成为京郊主要种植的叶类蔬菜。近年来,随着其种植面积的不断增加和设施种植模式的快速推广,细菌性软腐病发生日益频繁,成为快菜上一类毁灭性病害。目前,尚未见到有关快菜软腐病原的详细报道。
本研究针对2015年从北京不同区县快菜产地采集的软腐病株,结合病原菌形态学、生理生化及分子生物学手段对病原菌进行综合鉴定,以明确引发快菜软腐病致病菌种类及其生物学特性,为该病害的科学防治提供理论依据。
1 材料和方法
1.1 菌株来源及分离
本研究的软腐病菌采自北京昌平、通州、怀柔、大兴和房山区的快菜种植区。病原菌分离及回接试验参考《植病研究方法》(第3版)[9]。从接种48 h后发病组织上重新分离获得病原菌,并编号为KC-01~KC-19、KC-21~KC-30(表1),保存于-80 ℃备用。
表1 Pc菌株的致病力等级
Tab.1 Pathogenicity levels of the Pc strains isolated
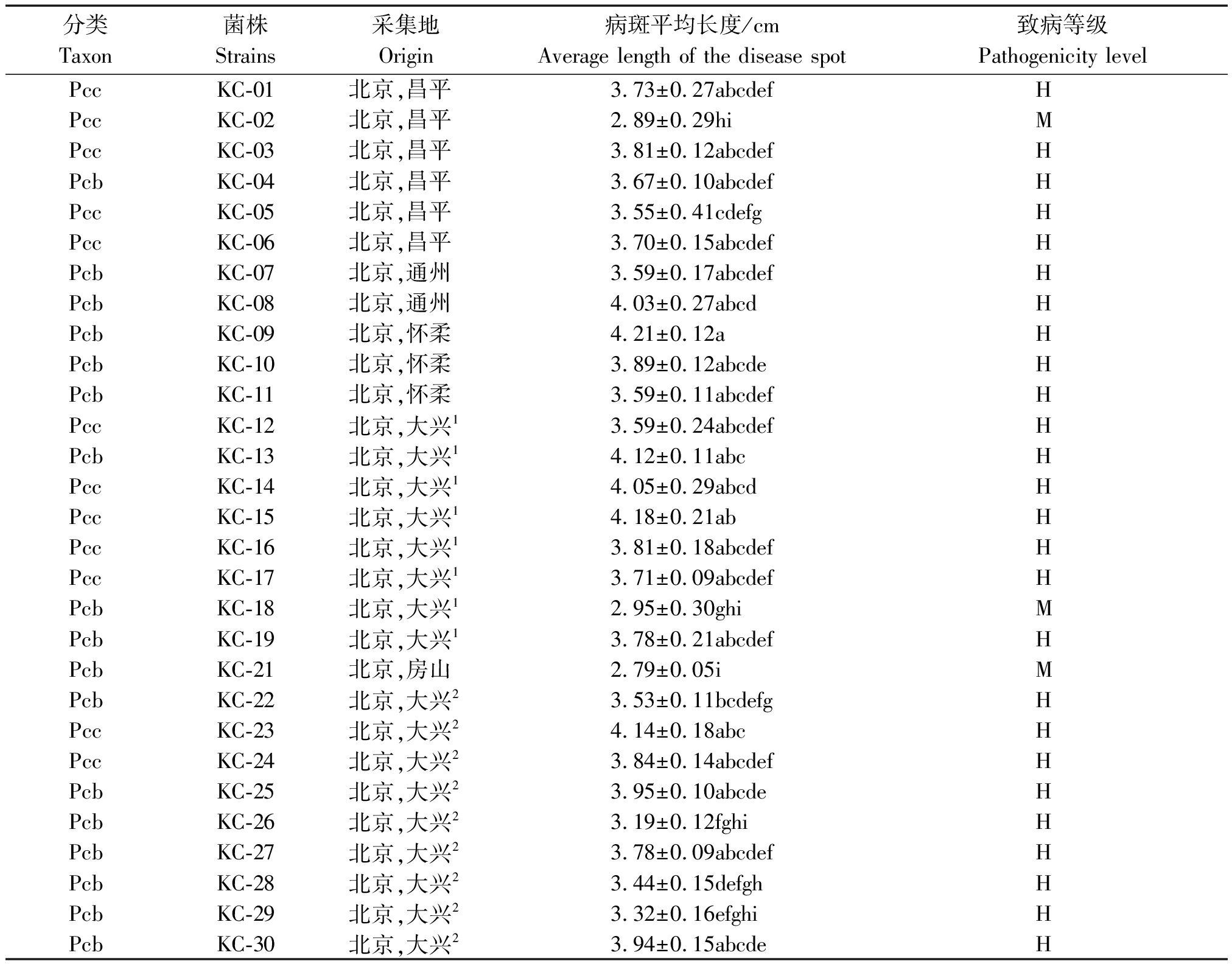
分类Taxon菌株 Strains采集地Origin病斑平均长度/cmAverage length of the disease spot致病等级Pathogenicity levelPccKC-01北京,昌平3.73±0.27abcdefHPccKC-02北京,昌平2.89±0.29hiMPccKC-03北京,昌平3.81±0.12abcdefHPcbKC-04北京,昌平3.67±0.10abcdefHPccKC-05北京,昌平3.55±0.41cdefgHPccKC-06北京,昌平3.70±0.15abcdefHPcbKC-07北京,通州3.59±0.17abcdefHPcbKC-08北京,通州4.03±0.27abcdHPcbKC-09北京,怀柔4.21±0.12aHPcbKC-10北京,怀柔3.89±0.12abcdeHPcbKC-11北京,怀柔3.59±0.11abcdefHPccKC-12北京,大兴13.59±0.24abcdefHPcbKC-13北京,大兴14.12±0.11abcHPccKC-14北京,大兴14.05±0.29abcdHPccKC-15北京,大兴14.18±0.21abHPccKC-16北京,大兴13.81±0.18abcdefHPccKC-17北京,大兴13.71±0.09abcdefHPcbKC-18北京,大兴12.95±0.30ghiMPcbKC-19北京,大兴13.78±0.21abcdefHPcbKC-21北京,房山2.79±0.05iMPcbKC-22北京,大兴23.53±0.11bcdefgHPccKC-23北京,大兴24.14±0.18abcHPccKC-24北京,大兴23.84±0.14abcdefHPcbKC-25北京,大兴23.95±0.10abcdeHPcbKC-26北京,大兴23.19±0.12fghiHPcbKC-27北京,大兴23.78±0.09abcdefHPcbKC-28北京,大兴23.44±0.15defghHPcbKC-29北京,大兴23.32±0.16efghiHPcbKC-30北京,大兴23.94±0.15abcdeH
注:大兴1.大兴区长子营镇南蒲洲村;大兴2.北京市大兴区农业技术示范站;不同小写字母代表显著性差异分析结果(P<0.05,Duncan);M.中致病力;H.高致病力。
Note:Daxing1.Nanpuzhou,Zhangziying,Daxing District;Daxing2.Agricultural Technology Demonstration Station,Daxing District;a-i.The different superscript letters indicate significant difference(P<0.05,Duncan);M.Medium pathogenicity;H. High pathogenicity.
1.2 致病力测定
选取健康的大白菜用于致病性分析。接种方法及致病力分级标准参照胡娜娜等[6]的方法。在同等条件下,设接种无菌0.9% NaCl生理盐水的植物组织为对照。接种后24 h测量病斑的长度,设置3次重复,取其平均值作为平均病斑长度。采用SPSS 19.0方差分析程序(ANOVA)对不同菌株间的致病力进行差异显著性分析。
1.3 菌株形态、Biolog微生物鉴定系统及生理生化特性分析
菌株形态观察、结晶紫果胶酸盐(Crystal violet pectate,CVP)、菌落在KB培养基上的荧光反应及7% NaCl和37 ℃培养、氧化还原酶和磷酸酶活性、明胶液化以及其他12种碳水化合物的利用参考《植物病原细菌鉴定实验指导》(第3版)[10]。每个菌株设3次重复,参试的对照菌株为Pcc ECC71[11]、Pco BCS7[12]和Pcb BC1,不接菌的培养基为空白对照。Biolog微生物鉴定参照田宇等[7]的方法。
1.4 特异性PCR扩增分析
将纯化后的29个菌株于液体LB培养基中28 ℃振荡培养过夜,使用Easy Pure Genomic DNA Kit(TRANSGEN)试剂盒提取细菌基因组DNA。本研究所利用的特异性引物包括Pc的果胶酸盐裂解酶基因(pectate-lyase,pelY)特异性引物Y1/Y2[13](5′-TTACCGGACGCCGAGCTGTGGCGT-3′/5′-CAGGAAGATGTCGTTATCGCGAGT-3′)和Pcb特异性引物BR1f/L1r[14](5′-GCGTGCCGGGTTTATGACCT-3′/5′-CAAGGCATCCACCGT-3′)。利用引物G1/L1(5′-GAAGTCGTAACAAGG-3′/5′-CAAGGCATCCACCGT-3′)扩增16S~23S rDNA ITS(Intergenic transcribed spacer),结合限制性内切酶Rsa Ⅰ(Ferments,USA)酶切,进行PCR-RFLP鉴定[15]。
1.5 16S rDNA和pmrA基因序列系统关系分析
采用16S rDNA引物Pe1/Pe2[6]和pmrA基因引物F0145/E2477[16]对上述菌株基因组DNA进行PCR扩增及测序。基于本研究中29个菌株与NCBI上相关菌株的16S rDNA和pmrA基因完整序列,利用软件MEGA 6.0分别构建UPGMA(The unweighted pair group method with arithmetic averages)系统发育树,获得各菌株间的遗传关系。
2 结果与分析
2.1 软腐病原菌形态特征
从北京不同区县采集的快菜软腐病病样(图1-A)中分离得到了29株菌株。经回接验证(图1-D),均可引发快菜软腐病。发病部位呈淡黄色半透明的水浸状病斑,病部逐渐扩展后呈浅褐色的黏稠状,具恶臭气味。病原菌在LB固体培养基上28 ℃培养24 h,菌落呈圆形、乳白色、半透明且边缘整齐(图1-B),在CVP上可产生杯状凹陷(图1-C)。病原菌为革兰氏阴性菌(图1-E),形态呈短杆状,两头稍钝圆,鞭毛周生(图1-F)。以上菌株形态特征与Pectobacterium一致[9]。

A. 快菜软腐病田间发病症状;B. LB固体培养基上菌落形态;C. 菌株在CVP平板上产生杯状凹陷;D. 回接48 h后活体快菜发病症状(左为接种菌液后发病植株,右为接种清水的对照植株);E. 革兰氏染色(G-);F. 鞭毛染色。
A. Soft rot symptoms of Chinese cabbage in the fields;B. Bacterial colonies on LB medium;C. Cavities formed on CVP medium;D. Soft rot symptoms on Chinese cabbage 48 h after inoculation(the left side was the infected plant inoculated with the bacteria solution and the right side was the control plant inoculated with water);E. Gram staining of the bacterial isolates(G-);F. Flagella staining of the bacterial isolates.
图1 快菜软腐症状和病原菌形态学特征
Fig.1 Soft rot symptoms on Chinese cabbage and phenotypic characteristics of the bacterial soft rot pathogens
2.2 软腐病菌生理生化特性
Biolog微生物自动化鉴定系统将29株菌株鉴定为Pc,进一步生理生化特征分析结果显示:所有菌株在过氧化氢酶反应中呈阳性,可使明胶液化,在磷酸酶、氧化酶反应中呈阴性,在KB培养基上生长不产生荧光,可利用纤维素二糖、蜜二糖产酸,不能利用菊糖、山梨醇、α-甲基葡糖苷产酸;同时也与Pco可利用山梨醇和α-葡糖苷产酸特性不同,符合Pcc和Pcb生理生化性状特征描述[14,17-20]。这些菌株的生理生化特性也存在一些差异(表2):菌株KC-03和KC-06不具备在37 ℃及7% NaCl条件下生长的能力;仅KC-05、KC-12、KC-17、KC-23和KC-24可微利用异麦芽酮糖、D-麦芽糖、D-阿拉柏糖醇;KC-07不能利用棉籽糖产酸以及菌株KC-01~03、KC-06和KC-07无法利用柠檬酸盐。
表2 Pc菌株生理生化特征
Tab.2 Physiological and biochemical characterizations of the strains
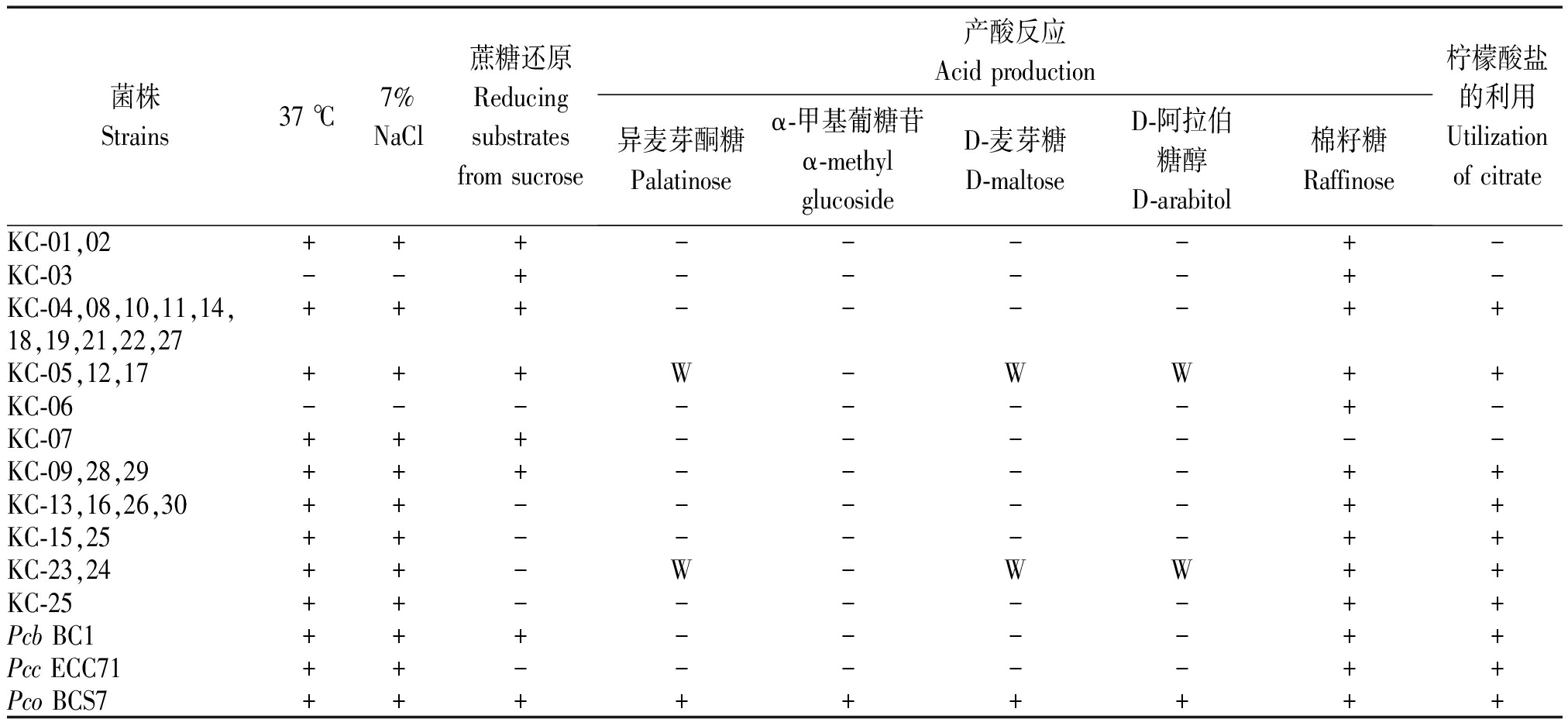
菌株Strains37 ℃7%NaCl蔗糖还原Reducingsubstratesfrom sucrose产酸反应Acid production 异麦芽酮糖Palatinoseα-甲基葡糖苷α-methyl glucosideD-麦芽糖D-maltoseD-阿拉伯糖醇D-arabitol 棉籽糖Raffinose柠檬酸盐的利用Utilizationof citrateKC-01,02+++----+-KC-03--+----+-KC-04,08,10,11,14,+++----++18,19,21,22,27KC-05,12,17+++W-WW++KC-06-------+-KC-07+++------KC-09,28,29+++----++KC-13,16,26,30++-----++KC-15,25++-----++KC-23,24++-W-WW++KC-25++-----++Pcb BC1+++----++Pcc ECC71++-----++Pco BCS7+++++++++
注:+. 阳性;-. 阴性;W. 微弱利用。
Note:+. Positive;-. Negative;W. Weak positive.
2.3 特异性引物PCR鉴定
利用Pc的Pel基因特异性引物Y1/Y2对29株菌株基因组DNA进行PCR扩增,均可得到预期片段大小为434 bp的产物(图2-A),而Pcb特异性引物BR1f/L1r在17株菌株及对照菌株BC1(Pcb)中均扩增出大小约322 bp的产物(图2-D);L1/G1引物16S~23S rDNA ITS区PCR扩增产物(图2-B)Rsa Ⅰ酶切结果显示,特征性带型可将29株菌株划分为Group Ⅰ~Ⅲ 3组(图2-C)。Group Ⅰ包含的17株菌株带型与对照菌株BC1(Pcb)相同,与Pcb特异性引物扩增结果一致;Group Ⅱ包含的4株菌株PCR-RFLP带型与ECC71(Pcc)带型相同;Group Ⅲ的8株菌株PCR-RFLP带型虽与本研究中的对照菌株均不相同,但与Toth等[15]报道的Pcc带型一致。
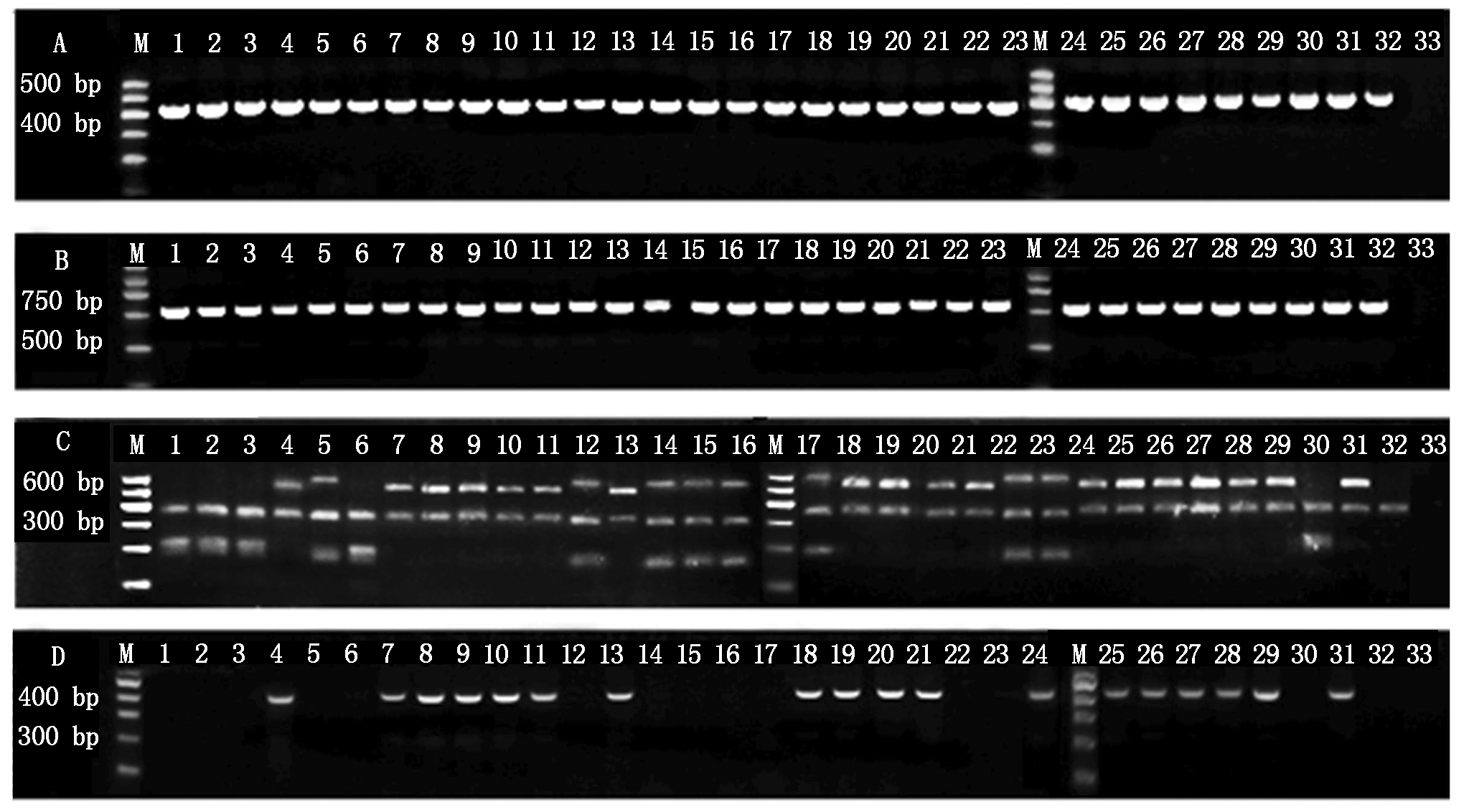
A. 引物 Y1/Y2;B. 引物G1/L1;C. Rsa Ⅰ限制性内切酶切ITS-RFLP带型;D. 引物BR1f/L1r。
1~29. 分离菌株KC-01~19、KC-21~30;30~32. 标准菌株Pcc ECC71、Pcb BC1和Pco BCS7;33. 阴性对照ddH2O。
A. Primers Y1/Y2;B. Primers G1/L1;C. ITS-RFLP patterns digested with Rsa Ⅰrestriction enzyme;D. Primers BR1f/L1r .1-29. Isolated strains KC-01-19 and KC-21-30;30-32. Standard strains Pcc ECC71,Pcb BC1,and Pco BCS7;33. Negative control ddH2O.
图2 Pc菌株特异性引物PCR和ITS-RFLP鉴定结果
Fig. 2 The identification of the Pc strains by PCR(using specific primers)and ITS-RFLP
2.4 16S rDNA和pmrA基因序列及系统发育树构建
对29株菌株的16S rDNA(GenBank登录号为KY021028-KY021056)和pmrA基因(KY020997-KY021015、KY021015-KY021026)进行PCR扩增测序,分别获得片段大小为666,1 530 bp的产物。基于pmrA基因完整序列构建的系统发育树中(图3),17株菌株与已发表的5株Pcb菌株形成了明显的Pcb类群,其余12株菌株则与已发表的5株Pcc菌株形成了Pcc类群,该结果与上述特异性引物和PCR-RFLP带型鉴定结果一致;因此,综合以上形态学、多种生理生化和分子生物学分析结果,确定了29株快菜细菌性软腐病菌株均属Pectobacterium carotovorum,其中17株为Pcb,12株为Pcc(表2)。pmrA基因序列系统发育树又进一步将所有的Pcc菌株分为2个亚类群(Ⅰ和Ⅱ):亚群Ⅰ包含ITS-RFLP带型中Group Ⅱ的4个菌株(KC-01、KC-02、KC-03和KC-06),亚群Ⅱ包含ITS-RFLP带型中Group Ⅱ的8株菌株(KC-05、KC-12、KC-14、KC-15、KC-16、KC-17、KC-23、KC-24)。
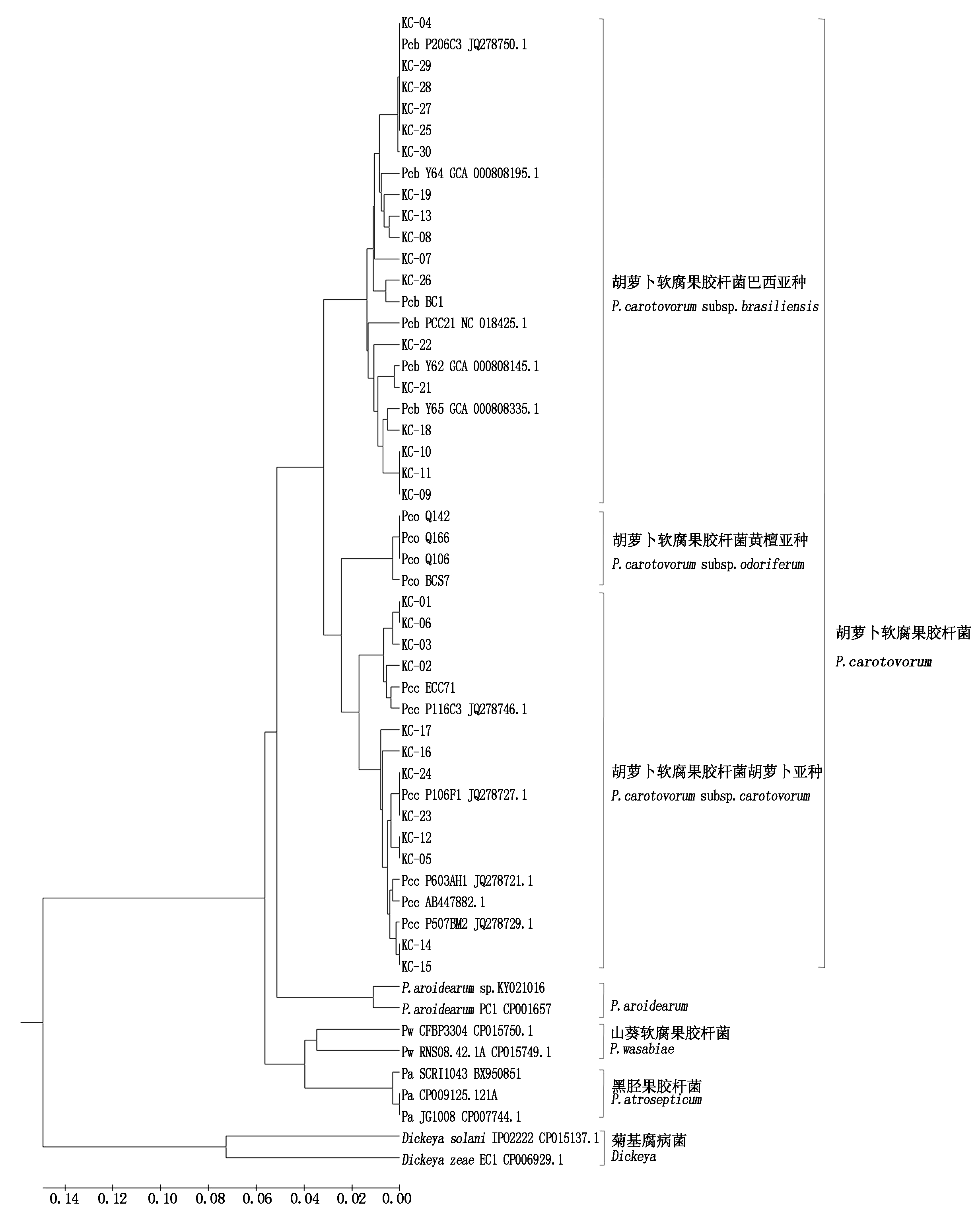
图3 基于pmrA基因完整序列构建的系统发育树
Fig.3 Phylogenetic tree based on the complete pmrA sequences
基于16S rDNA序列系统发育分析中(图4),4株菌株的聚类结果与上述鉴定结果(特异性引物PCR、PCR-RFLP和pmrA基因序列分析)不一致,其中,3株被上述结果鉴定为Pcb的菌株(KC-09、KC-11和KC-26)被聚类至Pcc类群;1株被上述结果鉴定为Pcb的菌株(KC-10),因扩增出2种类型(Type 1和Type 2)的16S rDNA序列被分别聚类至Pcb和Pcc类群。

2株Dickeya菌株为外群;KC-10菌株包含2种16S rDNA序列类型,KC-10 type 1归至Pcb类群,而KC-10 type 2归至Pcc类群;标记的4株菌株聚类情况与其在基于pmrA基因序列系统发育树中聚类情况不同。
Two Dickeya strains were used as the out-group;KC-10 strain had two types 16S rDNA sequences,KC-10 type 1 was belong to Pcb cluster,while KC-10 type 2 was belong to Pcc cluster;The four strains signed were placed differently from them in the pmrA sequences phylogenetic tree.
图4 基于16S rDNA完整序列构建的系统发育树
Fig.4 Phylogenetic tree based on the complete 16S rDNA sequences
2.5 软腐病菌致病力测定
29株Pectobacterium菌株接种离体大白菜24 h后,软腐病斑长度经单因素方差比较分析发现,不同菌株间致病力存在一定差异。除2株分别来自北京大兴和房山的Pcb菌株(KC-18和KC-21)和1株来自北京昌平的Pcc菌株(KC-02)表现出中等致病力外,其余菌株均表现出高致病力;不存在弱毒或无毒菌株(表1)。
3 讨论与结论
近年来,细菌性软腐病在叶类蔬菜上普遍发生[20-22],给叶菜产业造成严重的经济损失。本研究的29株病原菌株在形态学特征上符合果胶杆菌(Pectobacterium)的描述[10]。根据其生理生化特征将29株病原菌株确定为Pcc和Pcb。特异性引物PCR、ITS-RFLP和pmrA基因序列分析结果将这些菌株进一步鉴定为17株Pcb菌株和12株Pcc菌株。本研究对这些菌株也进行16S rDNA序列系统发育分析显示,其中4株菌株的聚类结果与上述鉴定结果(特异性引物PCR、PCR-RFLP和pmrA基因序列分析)不一致。有研究表明,16S rDNA高度保守性不能提供足够的区分度和支持率来进行软腐果胶杆菌亚种水平的遗传多样性分析[23],而pmrA基因是影响细菌胞外酶产生的一个重要单拷贝基因,存在于大部分的肠杆菌科(Enterobacteriaceae)细菌中,且其核酸序列在亚种中具有高度的保守性[24]。本研究中该基因序列分析可用于快菜软腐病原菌亚种水平的鉴定。另外,本研究中基于该序列构建的进化树将Pcc亚种菌株划分为不同亚群,其结果与ITS-RFLP带型分类结果一致。因此,pmrA 比16S rDNA更适用于果胶杆菌亚种内鉴定,结果准确、可靠。
与以往报道的典型Pcc菌株不同的是,本研究发现了9株非典型Pcc菌株,表现为不具备在37 ℃和7% NaCl条件下生长的能力或不能分解柠檬酸盐或可微利用D-阿拉伯糖醇、异麦芽酮糖醇和D-麦芽糖,但该结果与其他报道中非典型Pcc菌株特征相近[17,25-26]。根据ITS-RFLP和pmrA基因序列分析结果,本研究鉴定得到的12株Pcc菌株被进一步分为了2个亚群(亚群Ⅰ和亚群Ⅱ);而生理生化分析结果表明,亚群Ⅰ的菌株均不能利用柠檬酸盐,而亚群Ⅱ的菌株均能利用柠檬酸盐,由此说明2个亚群菌株间存在基因组和表型差异。此外,本研究对北京快菜软腐病原菌株的鉴定结果与此前对北京白菜类油菜(Brassica rapa subsp. chinensis)软腐病原菌株的报道[6]一致,均为Pcc和Pcb亚种,推测这2个亚种可能是引发北京地区白菜类蔬菜细菌性软腐病的优势种群。最后,为明确北京地区快菜软腐菌的致病力,本研究还对分离到的29株Pectobacterium菌株进行了致病力测定,结果显示,除3株菌株表现为中等致病力外,其余菌株均表现为强致病力,这与此前报道的北京地区油菜和芹菜上分离的软腐菌株大多表现出高致病力的研究结果一致[6-7]。
本研究通过对快菜细菌性软腐病菌形态学、多种生理生化及分子鉴定结果的综合分析,明确了引起北京地区快菜软腐病的主要致病菌为Pectobacterium carotovorum subsp. carotovorum和Pectobacterium carotovorum subsp. brasiliensis,且多数菌株具有强致病力。确认了pmrA基因序列分析在软腐果胶杆菌亚种鉴定中的准确性和适用性。本研究结果对快菜软腐病的综合防治具有重要意义。
参考文献:
[1] Davidsson P R, Kariola T, Niemi O, et al. Pathogenicity of and plant immunity to soft rot pectobacteria[J]. Front Plant Science,2013, 4:191.
[2] Nabhan S,Wydra K,Linde M,et al. The use of two complementary DNA assays,AFLP and MLSA,for epidemic and phylogenetic studies of pectolytic enterobacterial strains with focus on the heterogeneous species Pectobacterium carotovorum[J]. Plant Pathology,2012,61(3):498-508.
[3] Alvarado I C M,Michereff S J,Mariano R L R,et al. Characterization and variability of soft rot-causing bacteria in Chinese cabbage in north eastern Brazil[J]. Journal of Plant Pathology,2011,93(1):173-181.
[4] Shim H, Myung I S, Hong S K, et al. Outbreak of soft rot disease caused by Pectobacterium carotovorum subsp. carotovorum of Chinese cabbage followed by climate change in highlands of Korea[C]. Korea:Society for plant pathology in China Annual Academic Symposium, 2011: 218-221.
[5] Waleron M,Waleron K,Lojkowska E. First report of Pectobacterium carotovorum subsp. Brasiliense causing soft rot on potato and other vegetables in Poland[J]. Plant Disease,2015,99(9):1271.
[6] 胡娜娜,李 超,王 茜,等. 北京地区油菜软腐病病原菌的鉴定[J]. 微生物学报,2015,55(10):1253-1263.
[7] 田 宇,马亚丽,何付新,等. 北京地区芹菜细菌性软腐病菌鉴定及其致病力分析[J]. 植物病理学报,2016,46(4):433-442.
[8] 晋知文,宋加伟,谢学文,等. 芹菜细菌性软腐病病原的分离与鉴定[J]. 植物病理学报, 2016, 46(3): 304-312.
[9] 方中达. 植病研究方法[M]. 北京:农业出版社,1979:165-211.
[10] Schaad N W. Laboratory guide for identification of plant pathogenic bacteria[M]. St. Paul: APS Press , 2001.
[11] Mukherjee A,Cui Y,Ma W,et al. hexA of Erwinia carotovora ssp. carotovora strain Ecc71 negatively regulates production of RpoS and rsmB RNA,a global regulator of extracellular proteins,plant virulence and the quorum-sensing signal,N-(3-oxohexanoyl)-L-homoserine lactone[J]. Environmental Microbiology,2000,2(2):203-215.
[12] Zhu L,Xie H,Chen S,et al. Rapid isolation,identification and phylogenetic analysis of Pectobacterium carotovorum ssp. [J]. Journal of Plant Pathology,2010,92(2):479-483.
[13] Darrasse A,Priou S,Kotoujansky A,et al. PCR and restriction fragment length polymorphism of a pel gene as a tool to identify Erwinia carotovora in relation to potato diseases[J]. Applied and Environmental Microbiology,1994,60(5):1437-1443.
[14] Duarte V,de Boer S H,Ward L J,et al. Characterization of atypical Erwinia carotovora strains causing blackleg of potato in Brazil[J]. Journal of Applied Microbiology,2004,96(3):535-545.
[15] Toth I K,Avrova A O,Hyman L J. Rapid identification and differentiation of the soft rot erwinias by 16S-23S intergenic transcribed spacer-PCR and restriction fragment length polymorphism analyses[J]. Applied and Environmental Microbiology,2001,67(9):4070-4076.
[16] Kettani-Halabi M,Terta M,Amdan M,et al. An easy,simple inexpensive test for the specific detection of Pectobacterium carotovorum subsp. carotovorum based on sequence analysis of the pmrA gene[J]. BMC Microbiology,2013,13(1):176.
[17] Terta M,El Karkouri A,Mhand R,et al. Occurrence of Pectobacterium carotovorum strains isolated from potato soft rot in Morocco[J]. Cellular and Molecular Biology,2010,56(S1):OL1324-OL1333.
[18] Gardan L,Gouy C,Christen R,et al. Elevation of three subspecies of Pectobacterium carotovorum to species level:Pectobacterium atrosepticum sp. nov., Pectobacterium betavasculorum sp. nov. and Pectobacterium wasabiae sp. nov[J]. International Journal of Systematic and Evolutionary Microbiology,2003,53(Pt 2):381-391.
[19] Perombelon M C, van der Wolf J M. Methods for the detection and quantification of Erwinia carotovora subsp. atroseptica on potatoes suitable for commercial use: a laboratory manual[M]. Invergowrie, Dundee, Scotland: Scottish Crop Research Institute, 2002.
[20] Faquihi H,Terta M,Amdan M,et al. Phenotypic and genotypic diversity of Pectobacterium carotovorum subsp. carotovorum causing soft rot disease of potatoes in Morocco[J]. European Journal of Plant Pathology,2015,143(4):801-811.
[21] Meng X, Chai A, Li B, et al. Emergence of bacterial soft rot in cucumber caused by Pectobacterium carotovorum subsp. brasiliense in China[J]. Plant Disease, 2017, 101(2): 279-287.
[22] Queiroz M F, Gama M A S, Mariano R L R, et al. First report of soft rot in kale caused by Pectobacterium carotovorum subsp. brasiliensis in Brazil[J]. Plant Disease, 2017,101: 1542.
[23] Staley J T. The bacterial species dilemma and the genomic-phylogenetic species concept[J]. Philosophical Transactions of the Royal Society of London. Series B,Biological Sciences,2006,361(1475):1899-1909.
[24] Wösten M M,Groisman E A. Molecular characterization of the PmrA regulon[J]. The Journal of Biological Chemistry,1999,274(38):27185-27190.
[25] Nabhan S,De Boer S H,Maiss E,et al. Taxonomic relatedness between Pectobacterium carotovorum subsp. carotovorum,Pectobacterium carotovorum subsp. odoriferum and Pectobacterium carotovorum subsp. brasiliense subsp. nov[J]. Journal of Applied Microbiology,2012,113(4):904-913.
[26] Baghaee-Ravari S,Rahimian H,Shams-Bakhsh M,et al. Characterization of Pectobacterium species from Iran using biochemical and molecular methods[J]. European Journal of Plant Pathology,2011,129(3):413-425.