Drought is an important factors affecting yield and quality in many crops[1-2]. Variety improving can increase drought-resistance ability in some extent,furthermore,it is a most effective and economic method in reducing drought damage[3]. However,even the strongest resistant germplasm materials cannot meet production demand in some arid zone. Additionally,drought resistance is a quantitative trait controlled by multiple genes[4-6],thus,it is difficult by conventional breeding improvement to increase resistance to drought in crops. Many researchers carried out much work in drought-resistance breeding in previous years[7-8],but there is no evident progress. Transgenic technique can break genetic interval between different species,and make one species be transformed into any ideal gene derived from other species. From literature,it was found that some resistant genes to drought including SOD and DREB genes have successfully been transformed into crops such as rice (Oryza sativa L.),maize (Zea mays L.),etc.[9-10],but the results showed that the transgenic plants and their progeny could not meet agricultural production requirement in drought resistance. Therefore,it is necessary to develop and utilize the genes possessing stronger resistance to drought in agricultural breeding.
Trehalose is widely distributed in lower organism such as yeast,nematode,algae,simultaneously,and so on. It plays a key role in resistance to water stress for these biology[11-12]. Previous studies demonstrated that some plant seeds,yeast cell,fungal spores and many microorganism could maintain long-term survival when their cells lost partial or total water,and could reactivate even dehydration for decades,the reason is that abundant trehalose was accumulated within their cells[13-14].
Trehalose-6-phosphate synthase (TPS) gene is a very important gene related to trehalose synthesis in vivo,and according to literature,there were some studies on TPS gene cloning were reported. For example,Kaasen et al[15]cloned two TPS genes,named otsA and otsB in E. coli,in same year,Bell et al[16]isolated two TPS genes from Saccharomyces cerevisiae,named TPS1 and TPS2. Furthermore,some TPS genes were introduced into plant for improving its tolerance to drought. For instances,The TPS1 gene from yeast was transformed into tobacco by Holmström et al[17],and the result showed that the transgenic tobacco enhanced water retention in leaf;the two TPS genes ostA and otsB isolated from E. coli were introduced into tobacco by Pilon-Smits et al[18],and the transgenic tobacco showed better biology yield,photosynthetic rate and water retention in leaf than control under drought stress. Furthermore,Zhang et al[19] transformed a TPS gene from yeast into maize by Agrobacterium-mediated transformation method,and some transformed plants displayed resistance to drought stress in some extent.
Although some TPS genes derived from other species have already been applied for improving resistance to drought in maize,the resistance to drought could not meet agricultural production demand. Thus,selecting and utilizing an elite TPS gene resources from other specifies is necessary and significant in maize drought-resistance breeding work. In this study,a TPS gene sequence from Gossypium arboretum was redesigned and synthesized based on maize preferred codons,and its expression was also finished in this experiment. This aim is to obtain a gene resource which can be effectively applied in drought-resistance transgenic breeding program in maize.
1 Materials and Methods
1.1 Gene,vectors and engineering strains
In my study,the gene used for structural construction and expression analysis was from Gossypium arboretum (GenBank:EU750912.1). The vectors involved in this experiment,included a cloning vector pMD19-T (Simple),a prokaryotic expression vector pET30a(+),a plant expression vector pCAMBIA2300. The engineering strains included two competent E. coli strains BL21(DE3) and DH5α,one Agrobacterium strain LBA4404 and one yeast strain Pichia pastoris GS115.
1.2 Gene synthesis and sequence analysis
For the original gene sequence,it was designed based upon the preference sequence of maize by professional software,and the modified new gene named T-TPS was synthesized and cloned into the T cloning site of the plasmid pMD19-T (Simple),and the recombinant plasmid (named pT-vector-TPS) was transformed into JM109 strain (belonging to E. coli) throughout heat shocked method. Next,the positive plasmid pT-vector-TPS was screened throughout blue-white selection,and the T-TPS gene was sequenced.
1.3 Primer design,PCR amplification,expression plasmid construction
The T-TPS gene within the plasmid pT-vector-TPS was amplified by polymerase chain reaction (PCR) using two primers. Forward primer:5′-GAATTCATGGC
AAGCAGGTC-3′ (named LXH-F-E),and Reverse primer:5′-CGCAAGCTTTCACGCATTTG-3′ (named LXH-R-H). LXH-F-E and LXH-R-H possess endonuclease sites Hind Ⅲ and EcoRⅠat their 5′-end,respectively.
PCR (polymerase chain reaction) procedure referred the manual of DNA polymerase. After PCR amplification,the products were digested by Hind Ⅲ and EcoRⅠtogether,then,one part digested products was assembled into the multiple cloning site of the prokaryotic expression vector named pET30a(+),then the recombinant prokaryotic expression plasmid (named pET-TPS) was transformed into DH5α strain (belonging to E. coli) competent cell,and the positive clone was screened out by blue-white selection. Subsequently,the plasmid within the positive clone was extracted and the products were analyzed by double-enzyme digestion plus 1% agarose gel electrophoresis to identify the recombinant plasmid pET-TPS. The other part digested products of PCR products of pT-vector-TPS was constructed into a plant eukaryotic expression vector pCAMBIA-2300,the recombinant plasmid (named pCAMBIA-TPS) was transformed into TOP10 competent cell (belonging to E. coli) and identified by plasmid extraction,enzyme digestion and gel electrophoresis experiments. Finally,the right expression plasmid was extracted from positive TOP10 cell and transformed into Agrobacterium strain LBA4404,and single positive clone was selected and identified.
Additionally,the synthesized T-TPS gene was amplified by another two primers. Forward primer:LXH-F-E (same as above),and Reverse primer:LXH-R:5′-GCGGCCGCAAGCTTTCACGCATTTG-3′(named LXH-R-N). Primer LXH-R-N possessed endonuclease site NotⅠ at 5′-end.
The amplified T-TPS gene by the two primers was further constructed into Pichia pastoris expression vector pPIC9k by the two restriction endonuclease sites EcoRⅠand NotⅠ,and transformed into TOP10 competent cell belonging to E. coli,subsequently,the positive was verified by plasmid extraction,endonuclease digestion and gel electrophoresis. The expression plasmid harboring T-TPS gene was named pPIC9k-TPS.
1.4 Prokaryotic expression analysis of T-TPS gene
To identify whether T-TPS gene could be expressed expected protein in prokaryotic cell,the constructed expression plasmid pET-TPS was transformed into the competent cell BL21(DE3) (belonging to E. coli). The positive clone including pET-TPS expression plasmid was cultured in LB liquid medium. When the threshold value of 0.5 for OD600,IPTG (Isopropyl-β-D-Thiogalactoside) was added to the final concentration 0.2 mmol/L,at 37 ℃ for 3 h,then 1 mL sample was get out with micropipettor and centrifuged,and the deposit was suspended within 80 μL ddH2O and 20 μL 5×Loading Buffer. Afterwards,the 100 μL mixture was treated by boiling water bath for 10 min to disrupt cells,after centrifugation,the supernate was analyzed the target protein by SDS-PAGE.
1.5 Eukaryotic expression analysis of T-TPS gene
So as to ascertain whether the constructed gene could be expressed into expected target protein in eukaryotic cell,the pPIC9k-TPS plasmid was digested using endonuclease SacⅠ as linear treatment,then,the linear plasmid (named pPIC9k-TPS-LIN) was transformed into Pichia pastoris GS115 by eletroporation method,and then cultured by selection on solid medium,to select his+ transformants. The selected his+ transformants were further used for genomic DNA extraction and PCR amplification by the two primers LXH-F-E and LXH-R-N,and the PCR products was analyzed by agarose gel electrophoresis. Additionally,the selected his+ transformants were cultured by gradient screening with G418 (a neomycin analogues) to screen high-copy T-TPS gene clone. The selected clone was further used for induction culture by adding methanol in medium,induction time was designed 0,6,12,18,24,48 and 72 h. After induction culture,total proteins were extracted and separated on SDS-PAGE,and the target protein encoded by the TPS gene was analyzed. The specific procedure was based on Pichia pastoris expression manual.
2 Results and Analysis
2.1 Optimized gene and its sequence identification
The sequence of the designed and reconstructed T-TPS gene was seen in Fig.1. The gene had 2 586 bp in length,and its coding strand including 600 A,669 C,703 G and 614 T. The protein encoded by the gene is up to 861 amino acids in length and 96.97 ku for predicted protein molecular weight(Fig.2),ATG(1-3 nt) and TGA (2 584-2 586 nt) are as its initiation and stop codons,respectively. T-TPS gene was cloned into the vector pMD19-T (Simple) (Fig.3). Further sequence analysis showed that the T-TPS gene sequence agreed with the original designed sequence.

This DNA sequence is the coding strand of synthesized double-strand DNA.
Fig.1 The sequence of T-TPS gene

Fig.2 The amino acid sequence encoded by T-TPS gene
2.2 Construction and identification of expression plasmids
The constructed pET-TPS expression plasmid can express T-TPS gene in prokaryotic cell(Fig.4),with 8.0 kb in length. The plasmid pET-TPS was extracted from positive clone and digested with Hind Ⅲ and EcoRⅠ,and the digested fragments were analyzed by 1% agarose gel electrophoresis (Fig.5).
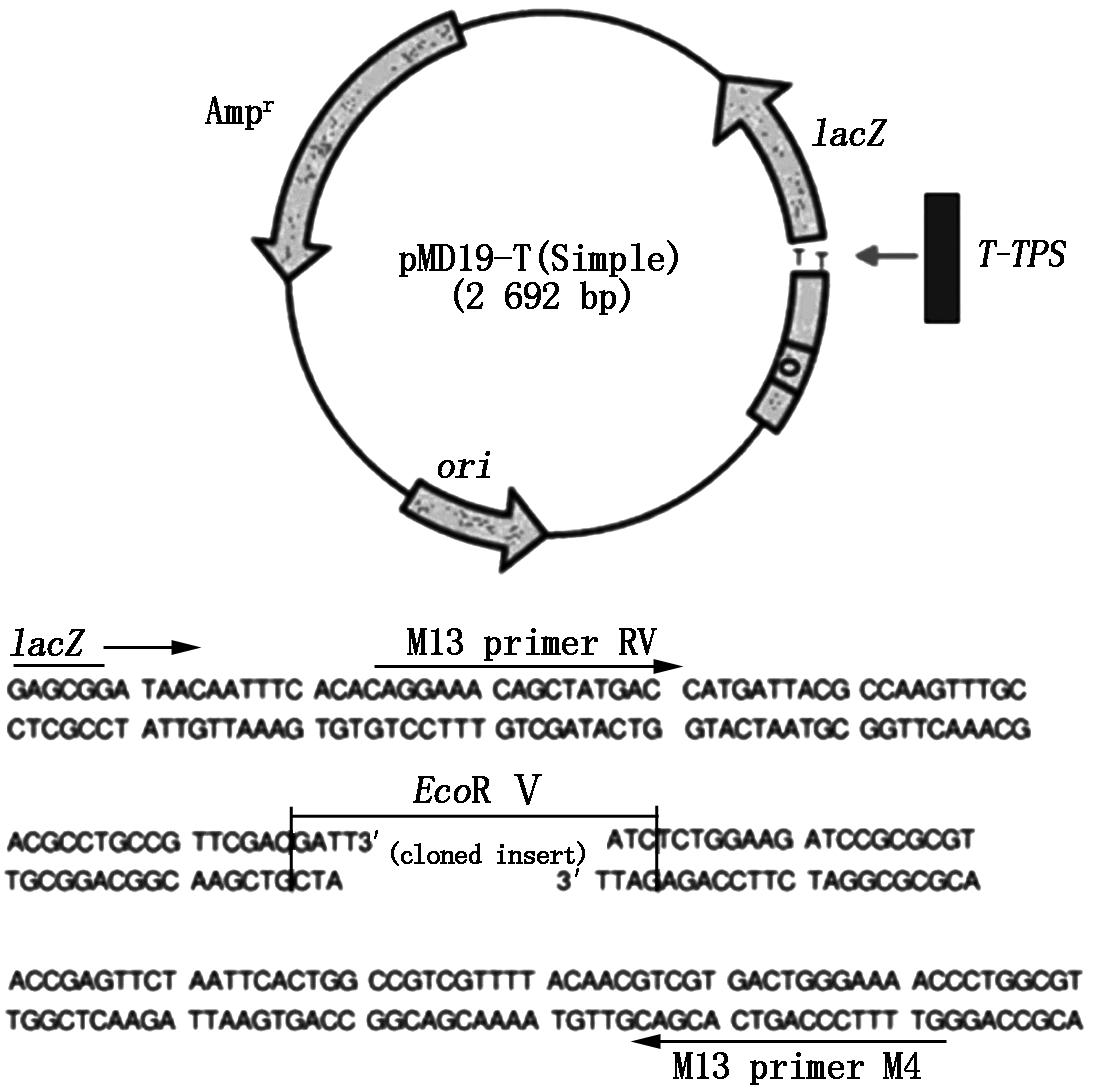
This plasmid including pMD19-T (Simple)(2 692 bp) and T-TPS gene (2 586 bp) .
Fig.3 The physical map of the plasmid pT-vector-TPS
The result demonstrated that the construction of pET-TPS was no problem.
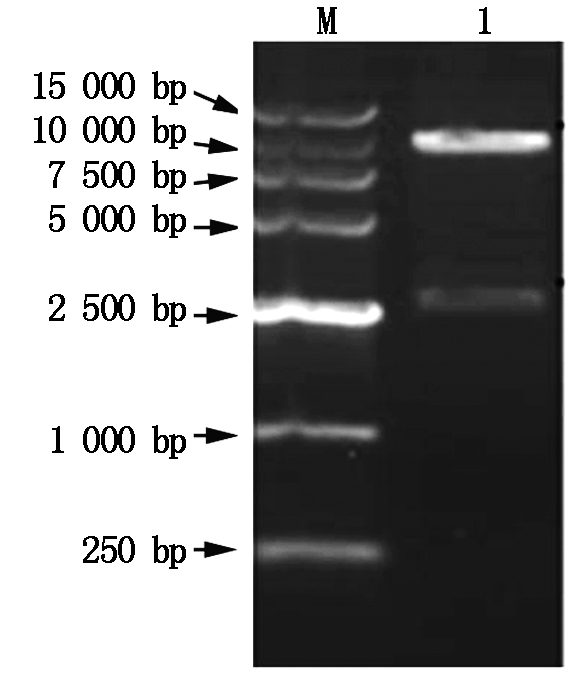
The constructed expression plasmid pCAMBIA-TPS (Fig.6) was isolated from positive clone and digested with Hind Ⅲ and EcoR Ⅰ,and the digested products were separated on 1% agarose gel electrophoresis. The result was seen in Fig.7,the construction of pCAMBIA-TPS agreed with our expectation. Furthermore,the plasmid pCAMBIA-TPS was transformed into Agrobacterium strain LBA4404,and the bacterial liquid was directly amplified by the two primers LXH-F-E and LXH-R-H using PCR technique,and the amplified products were identified by 1% agarose gel electrophoresis,the result was shown in Fig.8. The size of the bands of PCR products were in accord with T-TPS gene.

This plasmid including pET-30a(+) (5 422 bp) and T-TPS gene (2 586 bp).
Fig.4 The physical map of the expressing plasmid pET-TPS
For the constructed plasmid pPIC9k-TPS(Fig.9),after linear treatment with Sac Ⅰ,was transformed into Pichia pastoris GS115. Afterwards,the genomic DNA of positive yeast clone (his+) was extracted and the target gene (T-TPS) was amplified by PCR. The PCR result was shown in Fig.10,and the size of PCR products was accordant with our T-TPS gene.
2.3 Prokaryotic expression analysis of T-TPS gene
After induction expression for T-TPS gene in prokaryotic cell,the total protein was extracted and analyzed by SDS-PAGE,and the result was shown in Fig.11. The predicted molecular weight of the target protein should be 96.97 ku from amino acids sequence of T-TPS gene. From electrophoresis band on target location in the figure,it was easily found that T-TPS gene in BL21(DE3) cell was expressed efficiently under induction by IPTG for 3 h.
2.4 Eukaryotic expression analysis of T-TPS gene
For the screened out high-copy clone,was further induced to express target protein by adding methanol in different time,afterwards,the total protein was extracted and analyzed by SDS-PAGE,and the result was shown in Fig.12. Based on the electrophoresis band,it was obviously found that T-TPS gene was efficiently expressed into protein (96.97 ku) in Pichia pastoris GS115 cell under induction by adding methanol over 6 h,but for the control(CK),no target protein were found from the electrophoresis photograph. This result suggested that the target gene T-TPS could be induced to express the corresponding protein in eukaryotic cell.
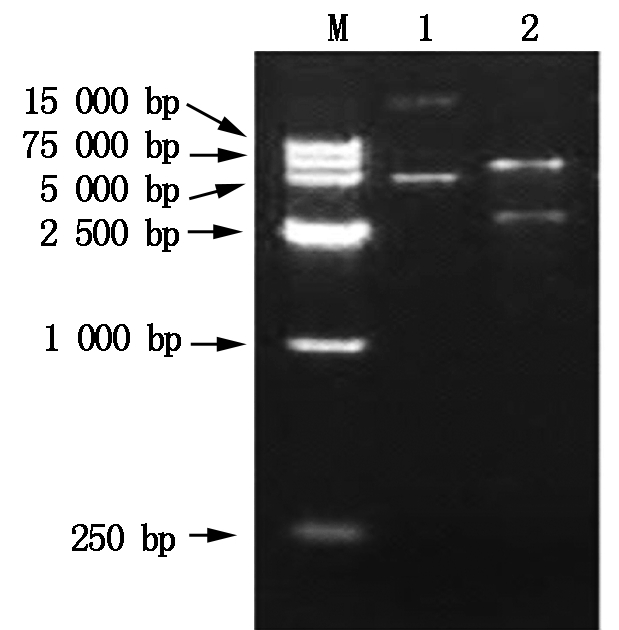
M.DNA Marker;1.pET-TPS plasmid;2.The products of digested pET-TPS by Hind Ⅲ and EcoRⅠ.
Fig.5 The 1% gel agarose electrophoresis photograph of the endonuclease digestion products of the recombinant expression plamid pET-TPS
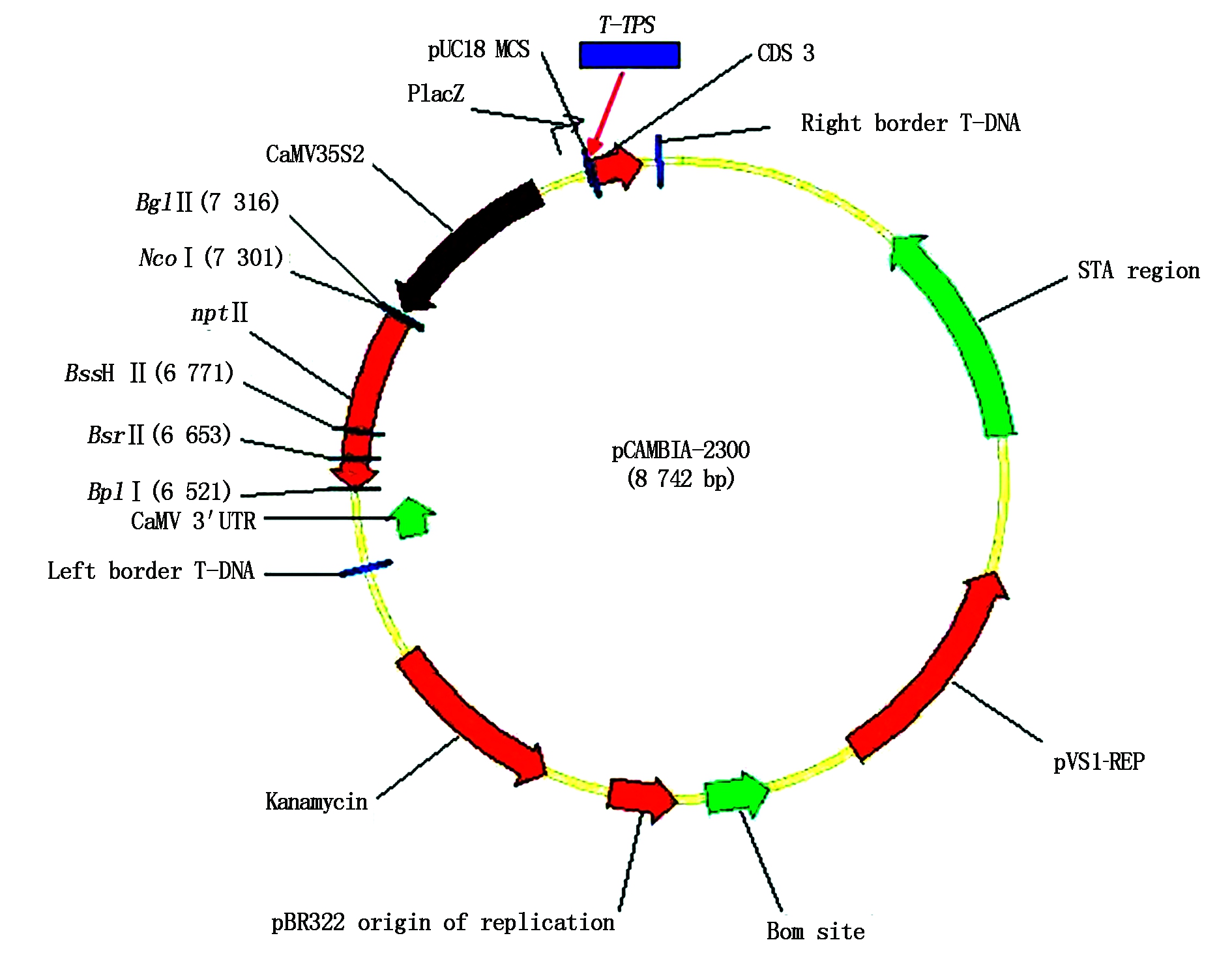
This plasmid including pCAMBIA-2300 (8 742 bp) and T-TPS gene (2 586 bp).
Fig.6 The physical map of the expression plasmid pCAMBIA-TPS
M.DNA Marker;1.The products of digested pCAMBIA-TPS by Hind Ⅲ and EcoRⅠ.
Fig.7 The 1% gel agarose electrophoresis photograph of the endonuclease digestion products of the recombinant expression plamid pCAMBIA-TPS
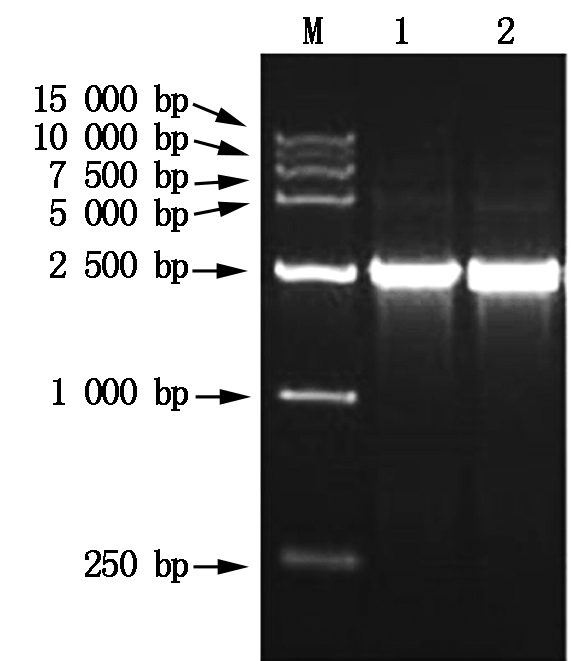
M.DNA Marker;1-2.PCR amplification products.
Fig.8 The 1% gel agarose electrophoresis photograph of PCR amplification products of the plasmid pCAMBI-TPS
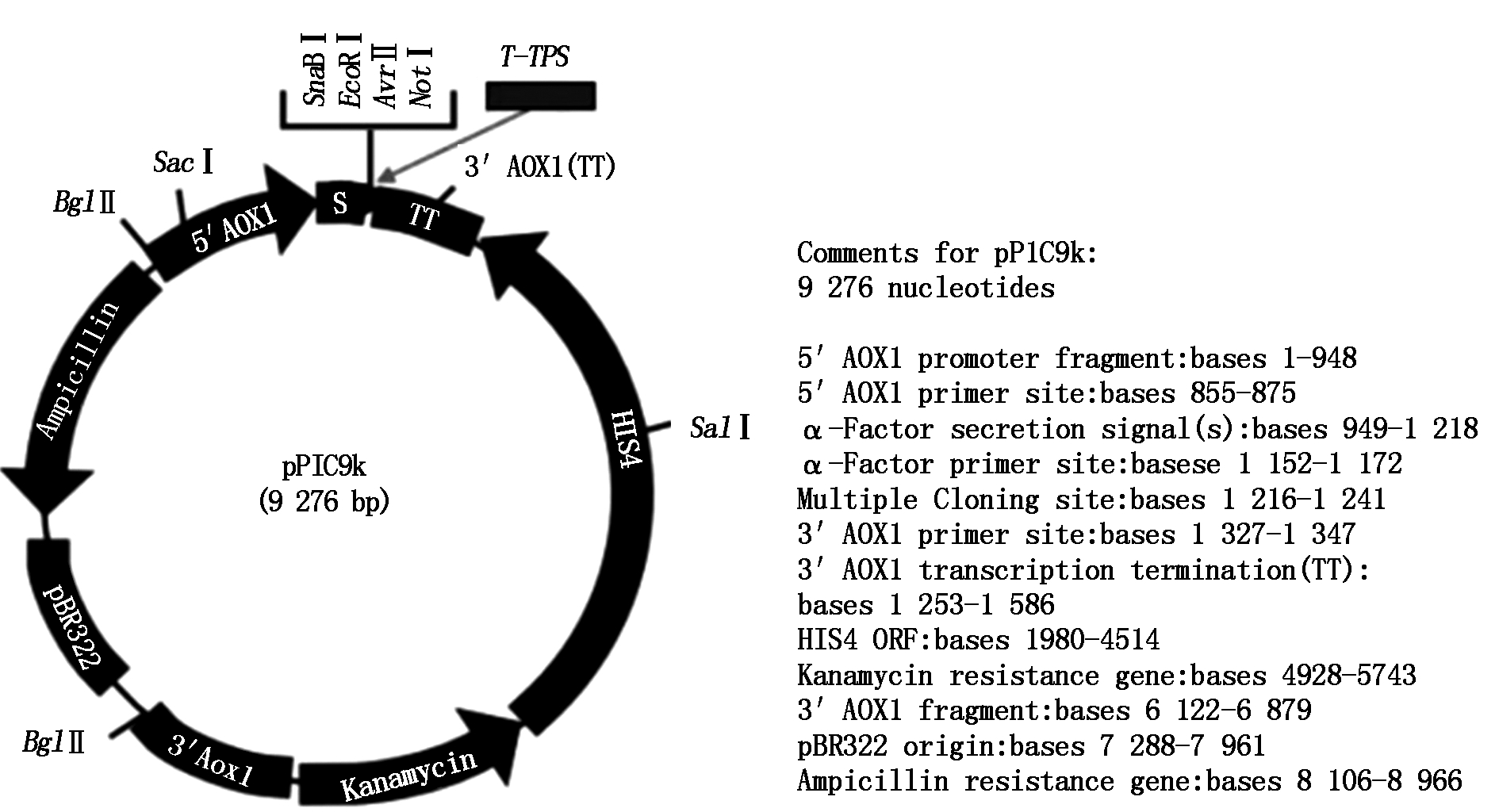
The plasmid consisting of vector pPIC9k (9 276 bp) and T-TPS gene (2 586 bp).
Fig.9 The physical map of the expression plasmid pPIC9k-TPS
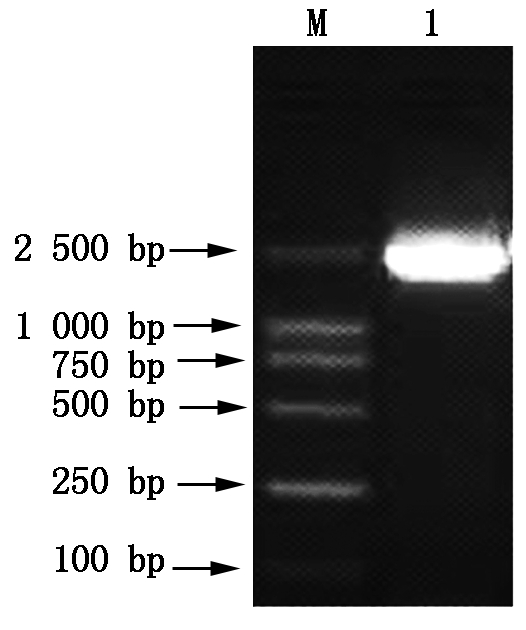
M.DNA Marker;1.PCR amplification products.
Fig.10 The 1% gel agarose electrophoresis photograph of PCR amplification products of the linear pPIC9k-TPS plasmid

M.Protein Marker;CK.Induction expression protein by IPTG for 3 h in BL21(DE3) cell that was transformed by pET30-30a(+) plasmid as control;1. Induction expression protein by IPTG for 3 h in for BL21(DE3) cell which was transformed by pET-TPS plasmid including T-TPS gene.
Fig.11 The SDS-PAGE photograph of induction expression protein in prokaryotic cell
3 Discussion
Drought is a major factor influencing plant growth and crop production[20]. Trehalose formation will be induced in many organisms that withstand drought,salt,heat or freeze stress. According to published literature,some "resurrection plants" such as Selaginella lepidophylla where it works as osmoprotectant during desiccation stress. It can also remain stable at elevated temperatures and low pH conditions[21]. To be mentioned,the overexpression of trehalose-6-phosphate synthase gene can promote high-level accumulation of trehalose in cell,this can further enhance plant′s resistance to drought [21-22].
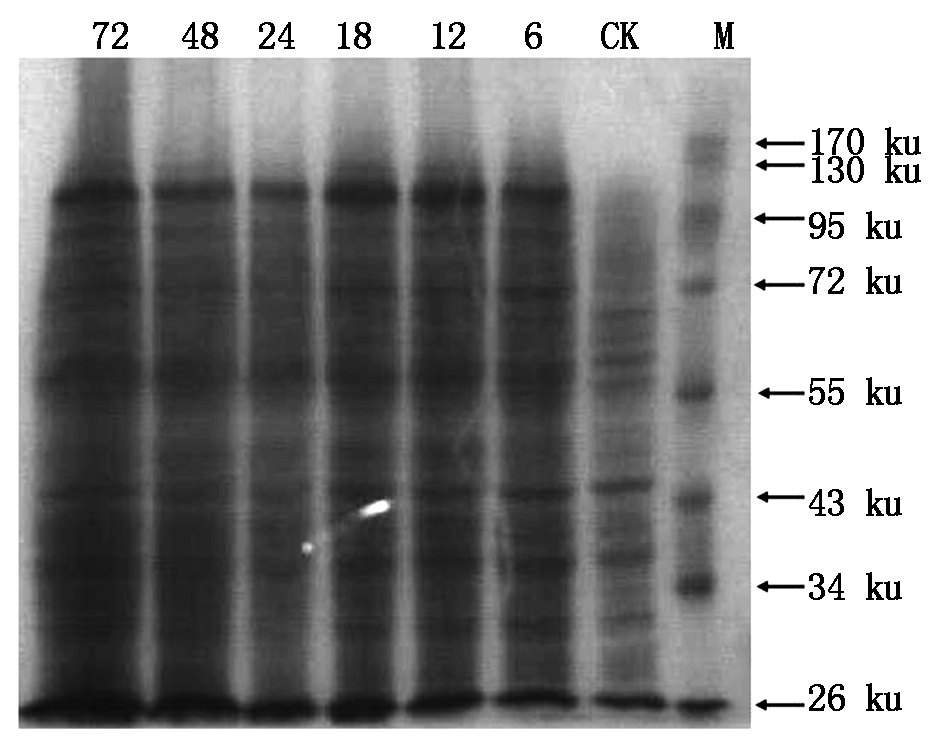
M.Protein Marker;CK.The expressed protein under no inducer methanol in Pichia pastoris GS115 cell including linear plasmid pPIC9k-TPS-LIN;6, 12, 18, 24, 48, 72.The expressed protein after induction by methanol for 6, 12, 18, 24, 48 and 72 h in Pichia pastoris GS115 cell including linear plasmid pPIC9k-TPS-LIN.
Fig.12 The SDS-PAGE photograph of the proteins extracted from eukaryotic cell
In this study,the reconstructed TPS gene spanning 2 586 bp coding fragment based on maize′s preference codons,and encoding 861 amino acids. According to previous studies[19-20,23],it should be a full-length cDNA sequence,thus,it can effectively express trehalose-6-phosphate synthase which catalyze trehalose synthesis in target cell after transformation in maize.
The new TPS gene was constructed into a prokaryotic expressing plasmid,and its expression products in E. coli cell was analyzed by SDS-PAGE,the result showed that it could effectively be expressed target protein. Additionally,the gene was cloned into a eukaryotic expressing vector,its expression products in yeast cell was estimated by SDS-PAGE,the result demonstrated the gene could effectively expressed into target protein in eukaryotic cell. All the experimental results were similar to previous studies[24-26]. Finally,the improved gene was constructed into a plant expression plasmid pCAMBIA-2300,and further transformed into Agrobacterium strain LBA4404. Thus,the obtained gene here can be directly transformed into maize cell by Agrobacterium-mediated transformation methods. These experiment results provided a gene resource in agriculture,which could be applied in maize drought-resistance breeding program.
参考文献:
[1] Feng X,Li W,Feng Y,et al. Identification of significant loci for drought resistance and root traits at seedling stage with a set of maize introgression lines [J]. Maydica,2013,58(3):231-237.
[2] Ghanbari A A,Mousavi S H,Mousapour GorJi A,et al. Combining abilities of grain yield and yield related traits in relation to drought tolerance in temperate maize breeding [J]. Turk J Field Crops,2015,20(2):203-212.
[3] Lin J,Fu F L,Jang W,et al. Cloning and functional analysis of trehalose-6-phosphate synthase gene from Selaginella pulvinata[J]. Hereditas,2010,32(5):498-504.
[4] Hao Z F,Li X H,Liu X L,et al. Meta-analysis of constitutive and adaptive QTL for drought tolerance in maize [J]. Euphytica,2010,174(2):165-177.
[5] Sandhu N,Singh A,Dixit S,et al. Identification and mapping of stable QTL with main and epistasis effect on rice grain yield under upland drought stress [J].Bmc Genetics,2014,15(1):63.
[6] Li C H,Sun B C,Li Y X,et al. Numerous genetic loci identified for drought tolerance in the maize nested association mapping populations [J]. Bmc Genetics,2016,17(1):894.
[7] Kanbar A,Shashidhar H E. Participatory selection assisted by DNA markers for enhanced drought resistance and productivity in rice (Oryza sativa L.) [J]. Euphytica,2011,178(1):137-150.
[8] Ghanbari A A,Mousav S H,Mousapour Gorji A,et al. Effects of water stress on leaves and seeds of bean (Phaseolus vulgaris L.) [J]. Turk J Field Crops,2013,18(1):73-77.
[9] Negi N P,Shrivastava D C,Sharma V A. Overexpression of CuZnSOD from Arachis hypogaea alleviates salinity and drought stress in tobacco [J]. Plant Cell Reports,2015,34(7):1109-1126.
[10] Jia X X,Li Y T,Qi E F,et al. Overexpression of the Arabidopsis DREB1A gene enhances potato drought-resistance [J]. Russian Journal of Plant Physiology,2016,63(4):523-531.
[11] Jun S S,Yang J Y,Choi H Y,et al. Altered physiology in trehalose-producing transgenic tobacco plants:Enhanced tolerance to drought and salinity stresses [J]. Journal of Plant Biology,2005,48(4):456-466.
[12] Akram N A,Noreen S,Noreen T,et al. Exogenous application of trehalose alters growth,physiology and nutrient composition in radish (Raphanus sativus L.) plants under water-deficit conditions [J]. Brazilian Journal of Botany,2015,38(3):431-439.
[13] Crowe J H,Hoekstra F A,Crowe L M. Anhydrobiosis [J]. Ann Rev Physiol,1992,54:579-599.
[14] Pramanik M H,Imai R. Functional identification of a trehalose 6-phosphate phosphatase gene that is involved in transient induction of trehalose biosynthesis during chilling stress in rice [J]. Plant Molecular Biology,2005,58(6):751-762.
[15] Kaasen I,Falkenberg P,Styrvold O B,et al. Molecular cloning and physical mapping of the otsBA genes,which encode the osmoregulatory trehalose pathway of Escherichia coli:evidence that transcription is activated by katF (AppR) [J]. J Bacteriol,1992,174(10):889-898.
[16] Bell W,Klaassen P,Ohnacker M,et al. Characterization of the 56-kDa subunit of yeast trehalose-6-phosphate synthase and cloning of its gene reveal its identity with the product of CIF1,a regulator of carbon catabolit einactivation [J]. Eur J Biochem,1992,209(3):951-959.
[17] Holmström K O,MäntyläE,Welin B,et al. Drought tolerance in tobacco [J]. Nature,1996,379:683-684.
[18] Pilon-Smits E A H,Terry N,Sears T,et al. Trehalose-producing transgenic tobacco plants show improved growth performance under drought stress [J]. J Plant Physiol,1998,152(4-5):525-523.
[19] Zhang Y Q,Dong C L,Yang L L,et al. Study on transformation of trehalose synthase gene TPS to maize and their drought resistance [J].Journal of Shanxi Agricultural Sciences,2016:44(1):1-4,39.
[20] Kosmas M A,Argyrokastritis A,Loukas M G,et al. Isolation and characterization of drought-related trehalose 6-phosphate-synthase gene from cultivated cotton (Gossypium hirsutum L.) [J]. Planta,2006,223(2):329-339.
[21] Wingler A. The function of trehalose biosynthesis in plants[J]. Phytochemistry,2002,60(5):437-440.
[22] An M Z,Tang Y Q,Mitsumasu K,et al. Enhanced thermotolerance for ethanol fermentation of Saccharomyces cerevisiae strain by overexpression of the gene coding for trehalose-6-phosphate synthase [J]. Biotechnology Letters,2011,33(7):1367-1374.
[23] Mu M,Lu X K,Wang J J,et al. Erratum to:genome-wide Identification and analysis of the stress-resistance function of the TPS(Trehalose-6-Phosphate Synthase)gene family in cotton [J].Bmc Genetics,2016,17(1):1-1.
[24] Wang G L,Zhao G,Feng Y B,et al. Cloning and comparative studies of seaweed trehalose-6-Phosphate synthase genes [J]. Marine Drugs,2010,8(7):2065-2079.
[25] Kondr k M,Ferenc M,Kalapos B,et al. Transcriptome analysis of potato leaves expressing the trehalose-6-phosphate synthase 1 gene of yeast [J].PLoS One,2011,6(8):e23466.
M,Ferenc M,Kalapos B,et al. Transcriptome analysis of potato leaves expressing the trehalose-6-phosphate synthase 1 gene of yeast [J].PLoS One,2011,6(8):e23466.
[26] Yang H L,Liu Y J,Wang C L,et al. Molecular evolution of trehalose-6-phosphate synthase(TPS)gene family in Populus,Arabidopsis and rice[J].PLoS One,2012,7(8):e42438.