一氧化氮(Nitric oxide,NO)是一种在植物生长发育及胁迫应答等方面发挥重要作用的气体信号分子[1-7]。低浓度的NO传导胁迫信号保护植物免受伤害,高浓度的NO具有很强的自由基活性,可以引起一些毒性效应。在细胞内NO基团可以转移到靶蛋白的巯基上,形成S-亚硝基硫醇(Nitrosthiols,SNOs),这个过程被称为S-亚硝基化(S-nitrosylation)[8]。越来越多的证据表明,蛋白质S-亚硝基化是NO发挥生物活性的主要机制[9-10]。
NO可以与体内主要的抗氧化物质谷胱甘肽(GSH)反应生成S-亚硝基谷胱甘肽(GSNO)。S-亚硝基谷胱甘肽还原酶(GSNOR),以前也被命名为还原型谷胱甘肽依赖型甲醛脱氢酶,属于醇脱氢酶Ⅲ类(ADH3)家族,普遍存在于所有生物中[11]。GSNOR通过促进GSNO代谢、降低NO的生物活性以间接调控蛋白亚硝基化的水平,进而参与植物生长发育、气孔信号转导、病原菌抵御、高温耐受性等多种生理过程[12-15]。低温、高温、连续光照、连续黑暗以及机械伤害处理下豌豆和向日葵中的GSNOR活性降低[16],砷胁迫下拟南芥中GSNOR活性增加,而镉胁迫下豌豆叶片GSNOR活性降低,并伴随NO和GSH含量降低[17],表明不同的植物受到的胁迫不同,GSNOR活性可能不同,而GSNOR在菠菜中是否存在以及其功能如何未见报道。
为了获得菠菜中的GSNOR基因序列,通过对已有物种的GSNOR序列分析,设计兼并引物,采用RT-PCR和RACE技术获得菠菜GSNOR基因全长cDNA序列,并命名为SoGSNOR,将SoGSNOR编码区重组到pET32a原核表达载体,诱导获得纯化蛋白并制备多克隆抗体,为进一步研究SoGSNOR在菠菜中的作用机制奠定基础。
1 材料和方法
1.1 试剂
基因克隆、电泳检测、目的片段回收、载体构建所用到的分子试剂为大连宝生物公司产品。蛋白纯化试剂盒为Promega公司产品;Western Blot所用试剂为康为世纪生物科技有限公司产品。本研究中所用引物均由上海捷瑞生物公司合成。
1.2 试验方法
1.2.1 SoGSNOR基因的克隆为了克隆菠菜的GSNOR基因,首先在NCBI网站比对其他物种GSNOR基因的同源序列,设计兼并引物,F1:ATGGC(T/A/G)AC(T/A)CAAGG(C/T)(C/A)A(A/G)GT(C/T/A)AT和R1:CCACC(A/G)AAAGC(T/A)GT(T/A)CC(T/C)TTCC,R2:CT(A/G)TA(G/A)TCAAC(T/A)CC(G/A/T)CCATC(T/A)GT。先用F1和R1进行第1轮扩增,反应条件为 94 ℃ 5 min;94 ℃ 30 s,50 ℃ 30 s,72 ℃ 60 s,共20个循环;最后一次循环结束后72 ℃延伸10 min。将扩增产物稀释10倍后用引物F1和R2进行第2轮扩增,反应条件为94 ℃ 5 min;94 ℃ 30 s,55 ℃ 30 s,72 ℃ 60 s, 30个循环;72 ℃延伸10 min。回收目的条带,连接到pMD18-T载体上,转化大肠杆菌,提取质粒进行PCR和酶切检测,测序获得基因的5′端序列。根据获得的基因序列,利用3′RACE获得基因的3′端序列。设计引物F2:TTTGGTCTTGGAACTGTAGG和F3:GTGGTGTATCTACGGGTCT,下游引物为B26:GACTCGAGTCGACATCGATTTTTTTTTTTTTTTT
TT。反应条件与5′端克隆一样进行巢式PCR,以F2 和B26引物扩增后的产物为模板,利用F3 和B26进行第2轮扩增。然后根据所得到的序列设计特异的全长和原核表达载体构建引物SoGSNOR-F-BamHⅠ:ggatccATGGCGACACAAGGCCAAG和SoGSNOR-R-XholⅠ:ctcgagTGGTTGATTTATTCATGCGC(小写字母为酶切位点)进行PCR扩增,得到SoGSNOR基因编码区序列。
1.2.2 原核表达载体pET32a-SoGSNOR的构建 将pMD18T-SoGSNOR质粒和原核表达载体pET32a质粒用BamH Ⅰ和XholⅠ进行双酶切,将回收的SoGSNOR片段与酶切后pET32a空载体用T4 DNA连接酶连接过夜,转化DH5α感受态细胞,筛选阳性克隆,测序,判断所插入的序列是否正确。
1.2.3 SoGSNOR重组蛋白的原核表达 测序正确后的pET32a-SoGSNOR质粒转化E.coli BL21感受态细胞,筛选阳性克隆,根据郭兆来等[18]方法进行原核表达,SDS-PAGE检测SoGSNOR重组蛋白的表达情况。
1.2.4 pET32a-SoGSNOR融合蛋白的纯化及多克隆抗体的制备 首先对蛋白表达条件进行优化,然后对大量培养的含pET32a-SoGSNOR的表达菌株进行诱导,离心回收沉淀,超声波破碎5 min,粗裂解液经10 000 r/min离心15 min,收集上清液,利用Ni2+ NTA亲和柱按照说明书纯化回收目的蛋白,最后进行SDS-PAGE分析。用纯化得到的融合蛋白为抗原,免疫昆明小鼠获得抗血清。
1.2.5 Western Blot检测SoGSNOR多克隆抗体 将诱导后的原核表达样品蛋白进行SDS-PAGE电泳后,经Biorad电转仪转膜进行Western Blot 操作,具体步骤根据郭兆来等[18]方法进行。
2 结果与分析
2.1 SoGSNOR的克隆及序列分析
根据其他物种的GSNOR基因序列,设计兼并引物,通过RT-PCR和3′RACE技术扩增菠菜SoGSNOR的编码框(ORF)序列(图1)。通过5′端的克隆,获得了780 bp的片段(图1-A),通过3′端的克隆,获得850 bp的片段。在拼接序列两端设计特异引物SoGSNOR-F-BamH Ⅰ和SoGSNOR-R-Xhol Ⅰ进行PCR扩增(图1-C),测序序列与基因的3′端和5′端拼接的结果一致,确定该3′端和5′端为同一基因的两段序列。
SoGSNOR cDNA序列全长1 378 bp,编码框1 140 bp,编码379个氨基酸(图2),预测分子量为40.7 ku,等电点为6.82。将序列在NCBI网站注册,获得GenBank登录号为KR381778。
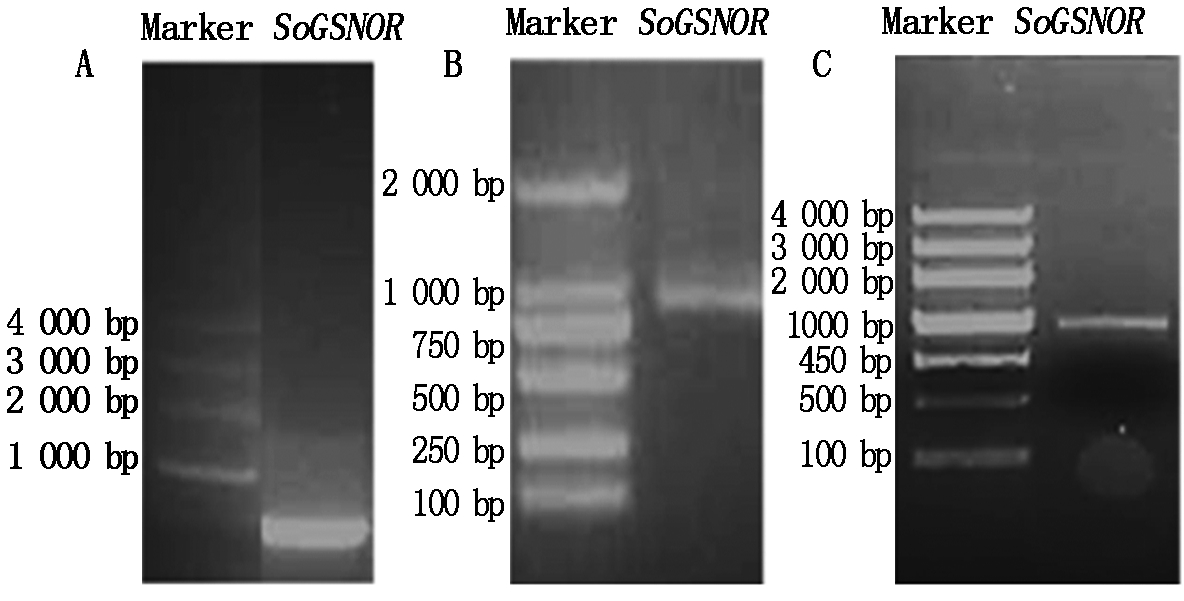
Marker.DNA ladder Marker.
图1 菠菜SoGSNOR的5′端序列(A)、3′端序列(B)和ORF(C)的扩增结果
Fig.1 The cloning of 5′ ends (A),3′ ends(B)and ORF(C) of SoGSNOR from spinach
2.2 pET32a-SoGSNOR原核表达载体的构建
提取pMD18T-SoGSNOR和原核表达载体pET32a
的质粒,利用BamH Ⅰ和Xhol Ⅰ进行双酶切、回收目的片段,连接,获得重组载体pET32a-SoGSNOR。将重组载体质粒进行BamH Ⅰ和Xhol Ⅰ双酶切,从图3可以看出,可切出目的条带。此外,测序结果也表明原核表达载体pET32a-SoGSNOR成功构建。
2.3 pET32a-SoGSNOR原核表达
将构建好的pET32a-SoGSNOR原核表达载体转化大肠杆菌BL21(DE3)。分别在16,28,37 ℃将含有pET32a-SoGSNOR菌株用1 mmol/L IPTG诱导0,2,4,6,8 h后,SDS-PAGE分析大肠杆菌总蛋白的表达情况。如图4所示,重组质粒pET32a-SoGSNOR在接近66.2 ku处表达出1条特异蛋白条带,且在16 ℃时,其表达量在0~8 h内随处理时间的增加而增加。

图2 SoGSNOR cDNA序列及其编码的氨基酸序列
Fig.2 Nucleotide and deduced amino acid sequences of the SoGSNOR
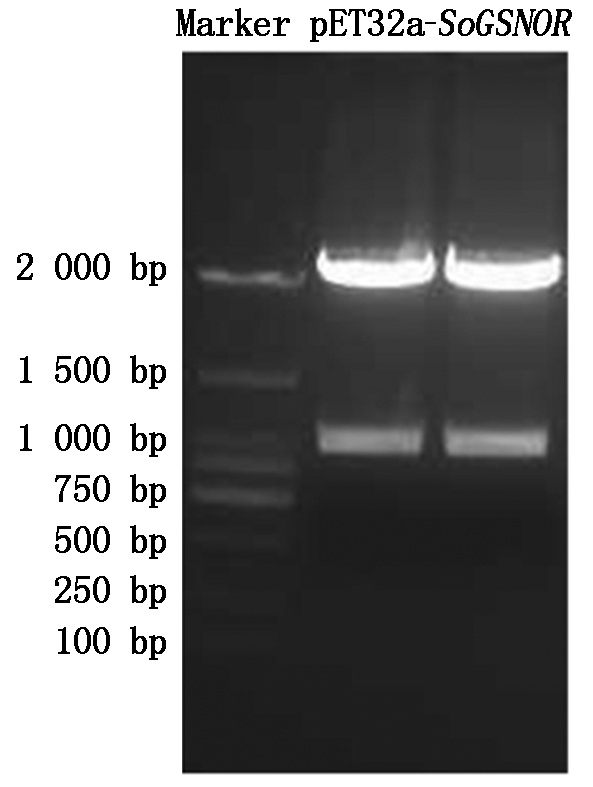
图3 pET32a-SoGSNOR重组质粒酶切鉴定
Fig.3 The restriction endonuclease digestof pET32a-SoGSNOR plasmid
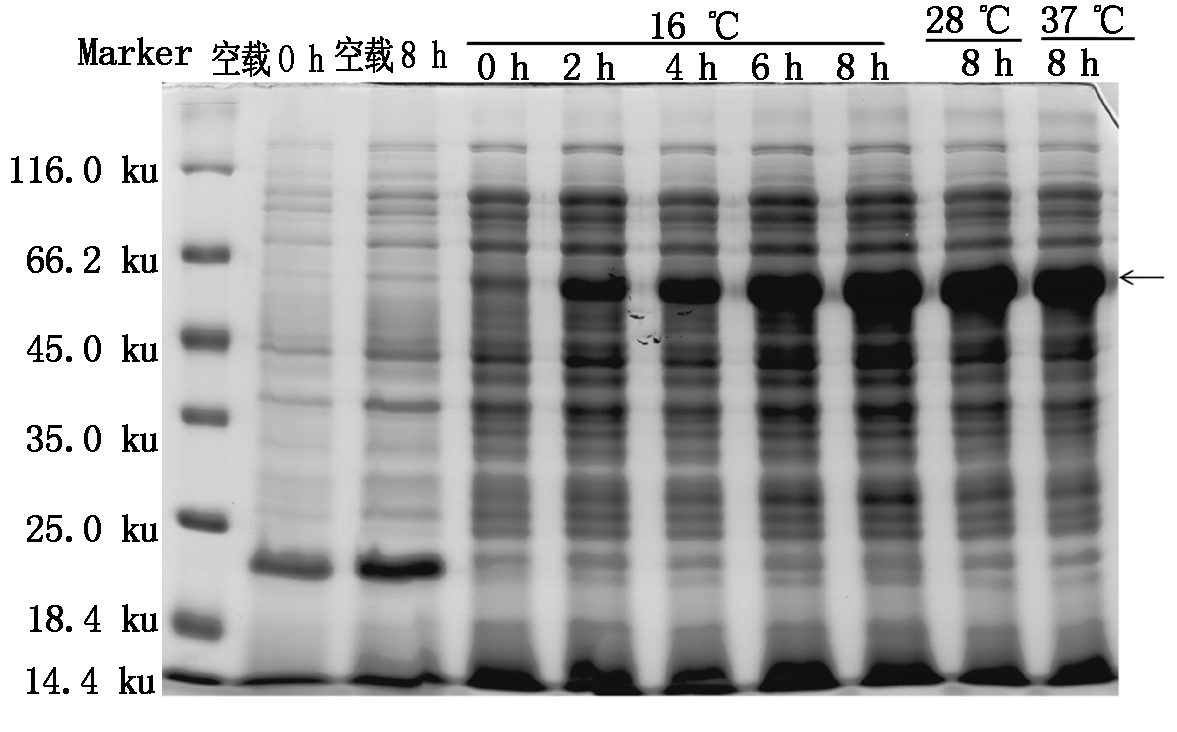
Marker.蛋白Marker。
Marker.Protein molecular weight Marker.
图4 SDS-PAGE检测pET32a-SoGSNOR融合蛋白的表达
Fig.4 The expression of pET32a-SoGSNOR wasanalyzed by SDS-PAGE
2.4 pET32a-SoGSNOR原核表达蛋白的纯化
以16 ℃为诱导温度,用1 mmol/L IPTG诱导扩大培养的pET32a-SoGSNOR菌株后超声波处理,分别收集上清液和沉淀。SDS-PAGE分析表明,上清液和沉淀中均有目的蛋白的表达。利用Ni2+NTA亲和柱对上清液进行纯化,用10,200 mmol/L的咪唑洗脱液洗脱,如图5所示,SDS-PAGE检测发现200 mmol/L的咪唑洗脱液洗脱后,有1条单一条带,大小约为65 ku,表明获得了纯化的SoGSNOR蛋白。
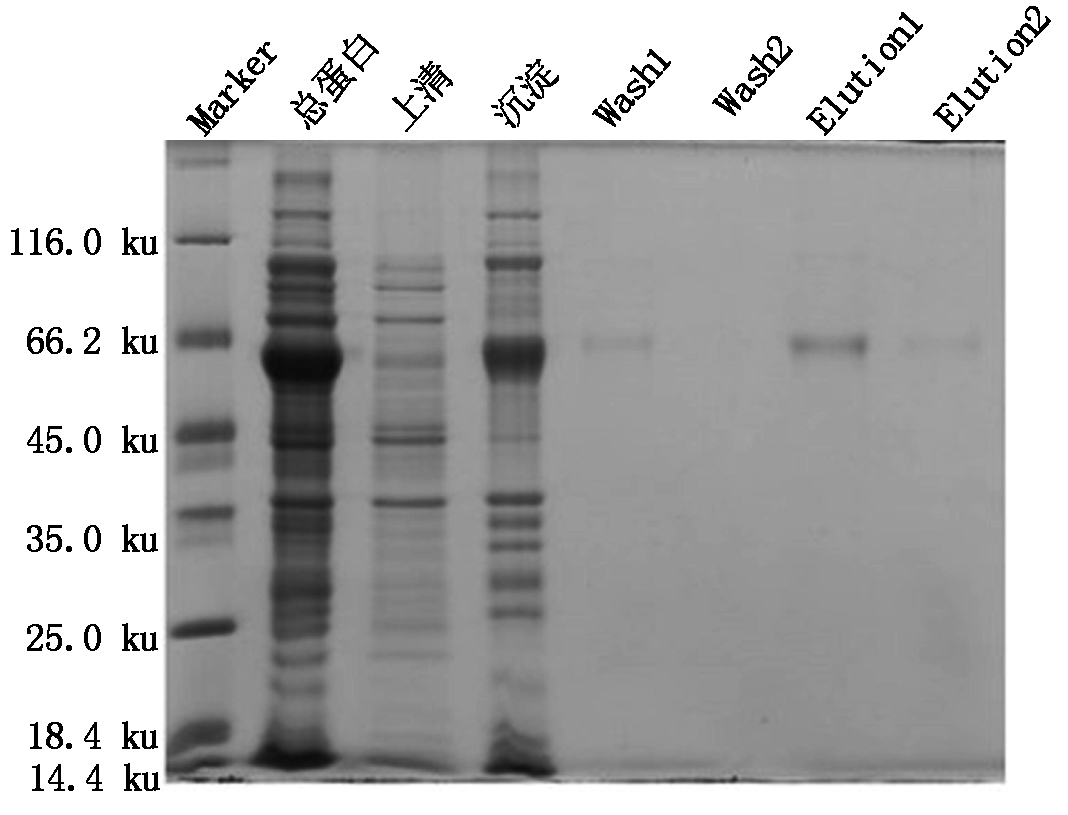
Marker.蛋白Marker;Wash1.第1次10 mmol/L咪唑洗脱;Wash2.第2次10 mmol/L咪唑洗脱;Elution1.第1次200 mmol/L咪唑洗脱;Elution2.第2次200 mmol/L咪唑洗脱。
Marker.Protein molecular weight Marker; Wash1,2.The fraction was eluted with 10 mmol/L imidazole Buffer for the first and second time; Elution 1,2.The fraction was eluted with 200 mmol/L imidazole Buffer for the first and second time.
图5 pET32a-SoGSNOR融合蛋白的纯化分析
Fig.5 The analysis of purified SoGSNOR fusion protein
2.5 Western Blot检测SoGSNOR多克隆抗体
为了检测SoGSNOR抗体的特异性和灵敏度,对pET32a-SoGSNOR原核表达菌体总蛋白、纯化的蛋白进行Western Blot检测(图6),结果显示均出现一条条带,大小与蛋白一致。多克隆抗体的效价较高,抗体稀释倍数可以达到1∶10 000,为SoGSNOR蛋白功能研究提供了可靠的抗体,可以用于后续试验。
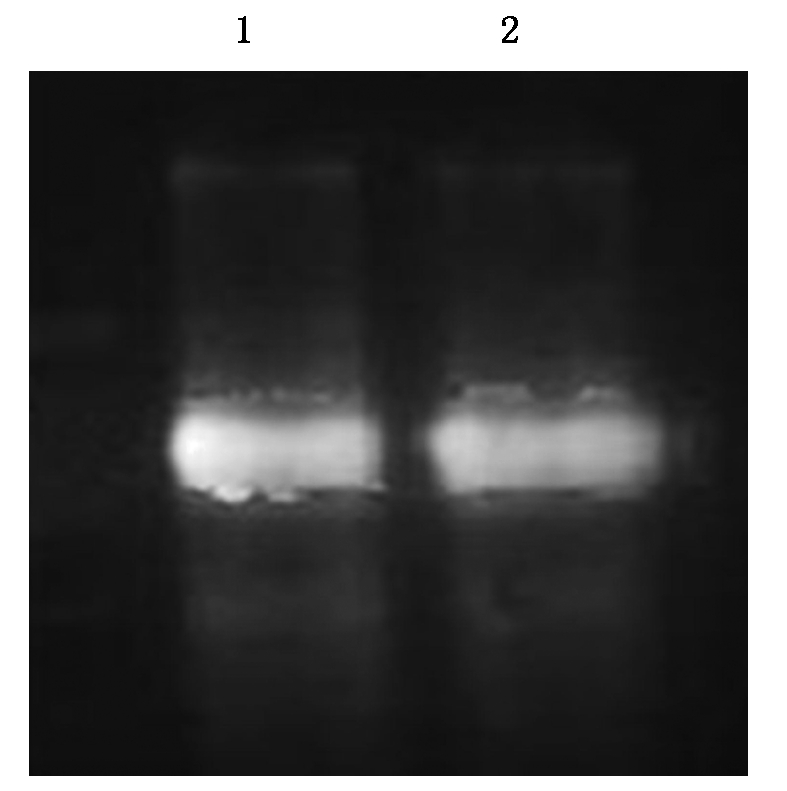
1.原核表达总蛋白;2.纯化后的原核表达蛋白。
1.The crude protein of pET32a-SoGSNOR;2.The purified protein of pET32a-SoGSNOR.
图6 SoGSNOR小鼠抗体的Western Blot检测
Fig.6 Western Blot analysis of theSoGSNOR polyclonal antiserum
3 讨论
GSNOR在植物界广泛存在,前人已经在拟南芥[11]、豌豆[17,19]、玉米[20]、水稻[21]、向日葵[22]、辣椒[23]和番茄[15]等发现GSNOR。李国钧[24]分别从水稻和番茄中克隆GSNOR基因OsGSNOR1和LeGSNOR1。马沅和陈放[25]克隆了麻风树JcGSNOR的全长序列,发现其在麻风树根、茎、叶及种子中均有表达,并在各部位持续表达可能是为维持体内NO在无害水平。本试验利用RT-PCR和RACE技术首次获得菠菜GSNOR基因的全长序列,编码框1 140 bp,编码379个氨基酸。
GSNOR参与植物生长发育、抵御生物和非生物胁迫等多种生理过程。拟南芥GSNOR完全缺失突变体表现出极度缺乏侧根原基,且回补系能完全恢复突变体的侧根表型[26]。番茄GSNOR调控的氧化还原信号控制番茄对碱胁迫的应答[27]。盐胁迫下向日葵中S-亚硝基还原酶活性降低[28]。初丛[29]研究发现烟草属的GSNOR1是烟草细胞死亡的负调控因子。GSNOR介导的去亚硝基化改变生长素信号和生长素的运输,是gsnor1-3突变体植物表型的主要原因[30]。菠菜GSNOR的表达特性和功能需要进一步研究。
大肠杆菌原核表达系统制备重组蛋白质具备很多优点,已被广泛应用于植物蛋白表达研究中[31]。本研究在大肠杆菌中高效表达了SoGSNOR蛋白,通过Ni2+ NTA亲和层析法获得纯化的目的蛋白,将该蛋白免疫小鼠制备了多克隆抗体。利用Western Blot分析发现,制备的多克隆抗体可以和抗原特异结合,表明成功制备了SoGSNOR的多克隆抗体,为后续进一步研究SoGSNOR的生物学功能奠定了基础。
参考文献:
[1] Beligni M V,Lamattina L.Nitric oxide stimulates seed germination and de-etiolation,and inhibits hypocotyl elongation,three light-inducible responses in plants[J].Planta,2000,210(2):211-215.
[2] He Y,Tang R H,Hao Y,et al.Nitric oxide represses the Arabidopsis floral transition[J].Science,2004,305(5692):1968-1971.
[3] Manoli A,Begheldo M,Genre A,et al.NO homeostasis is a key regulator of early nitrate perception and root elongation in maize[J].Journal of Experimental Botany,2014,65(1):185-200.
[4] Ziogas V,Tanou G,Belghazi M,et al.Roles of sodium hydrosulfide and sodium nitroprusside as priming molecules during drought acclimation in citrus plants[J].Plant Molecular Biology,2015,89(4/5):433-450.
[5] Silveira N M,Hancock J T,Frungillo L A,et al.Evidence towards the involvement of nitric oxide in drought tolerance of sugarcane[J].Plant Physiology and Biochemistry,2017,115:354-359.
[6] Yun B W,Skelly M J,Yin M H,et al.Nitric oxide and S-nitrosoglutathione function additively during plant immunity[J].New Phytologist,2016,211(2):516-526.
[7] Chen X,Tian D,Kong X,et al.The role of nitric oxide signalling in response to salt stress in Chlamydomonas reinhardtii[J].Planta,2016,244(3):651-669.
[8] Liu L M,Hausladen A,Zeng M,et al.A metabolic enzyme for S-nitrosothiol conserved from bacteria to humans[J].Nature,2001,410(6827):490-494.
[9] Besson-Bard A,Pugin A,Wendehenne D.New insights into nitric oxide signaling in plants[J].Annual Review of Plant Biology,2008,59:21-39.
[10] Lin A,Wang Y,Tang J,et al.Nitric oxide and protein S-nitrosylation are integral to Hydrogen peroxide-induced leaf cell death in rice[J].Plant Physiology,2012,158(1):451-464.
[11] Martinez M C,Achkor H,Persson B,et al.Arabidopsis formaldehyde dehydrogenase-Molecular properties of plant class Ⅲ alcohol dehydrogenase provide further insights into the origins,structure and function of plant class P and liver class Ⅰ alcohol dehydrogenases[J].European Journal of Biochemistry / FEBS,1996,241(3):849-857.
[12] Ticha T,Cincalova L,Kopecny D,et al.Characterization of S-nitrosoglutathionereductase from Brassica and Lactuca spp.and its modulation during plant development[J].Nitric Oxide,2017,68:68-76.
[13] Wang P C,Du Y Y,Hou Y J,et al.Nitric oxide negatively regulates abscisic acid signaling in guard cells by S-nitrosylation of OST1[J].Proceedings of the National Academy of Sciences of the United States of America,2015,112(2):613-618.
[14] 张 扬.番茄S-亚硝基谷胱甘肽还原酶基因SlGSNOR沉默对番茄高温抗性的影响[D].杭州:浙江大学,2013.
[15] Kubienová L,Kope![]() ny D,Tylichová M,et al.Structural and functional characterization of a plant S-nitrosoglutathionereductase from Solanum lycopersicum[J].Biochimie,2013,95(4):889-902.
ny D,Tylichová M,et al.Structural and functional characterization of a plant S-nitrosoglutathionereductase from Solanum lycopersicum[J].Biochimie,2013,95(4):889-902.
[16] Chaki M,Valderrama R,Fernández-Ocaňa A M,et al.Mechanical wounding induces a nitrosative stress by down-regulation of GSNO reductase and an increase in S-nitrosothiols in sunflower(Helianthus annuus)seedlings[J].Journal of Experimental Botany,2011,62(6):1803-1813.
[17] Barroso J B,Corpas F J,Carreras A,et al.Localization of S-nitrosoglutathione and expression of S-nitrosoglutathionereductase in pea plants under cadmium stress[J].Journal of Experimental Botany,2006,57(8):1785-1893.
[18] 郭兆来,白学贵,严金平,等.菠菜SoHb基因的原核表达及功能分析[J].中国生物工程杂志,2015,35(4):54-59.
[19] Shafqat J,Elahmad M,Danielsson O,et al.Pea formaldehyde-active class Ⅲ alcohol dehydrogenase:Common derivation of the plant and animal forms but not of the corresponding ethanol-active forms (classes Ⅰ and P) [J].Proceedings of the National Academy of Sciences of the United States of America,1996,93(11):5595-5599.
[20] Fliegmann J,Sandermann H J.Maize glutathione-dependent formaldehyde dehydrogenase cDNA:a novel plant gene of detoxification[J].Plant Molecular Biology,1997,34(6):843-854.
[21] Dolferus R,Osterman J C,Peacock W J,et al.Cloning of the arabidopsis and rice formaldehyde dehydrogenase genes:implications for the origin of plant ADH enzymes[J].Genetics,1997,146(3):1131-1141.
[22] Chaki M,Fernandez-Ocana A M,Valderrama R A,et al.Involvement of reactive nitrogen and oxygen species (RNS and ROS) in sunflower mildew interaction[J].Plant and Cell Physiology,2009,50(2):265-279.
[23] Airaki M,Leterrier M,Mateos R M,et al.Metabolism of reactive oxygen species and reactive nitrogen species in pepper (Capsicum annuum L.) plants under low temperature stress[J].Plant Cell and Environment,2012,35(2):281-295.
[24] 李国钧.水稻和番茄S-亚硝基谷胱甘肽还原酶基因的克隆鉴定与生化功能分析[D].杭州:浙江大学,2007.
[25] 马 沅,陈 放.麻疯树亚硝基谷胱甘肽还原酶(JcGSNOR)的克隆与功能分析[J].四川大学学报:自然科学版,2013,50(6):1355-1362.
[26] 孙艳艳.GSNOR在拟南芥侧根发育中的调控机理[D].兰州:兰州大学,2015.
[27] Gong B,Wen D,Wang X,et al.S-nitrosoglutathione reductase-modulated redox signaling controls sodic alkaline stress responses in Solanum lycopersicum L.[J].Plant & Cell Physiology,2015,56(4):790-802.
[28] Jain P,Von T C,Lindermayr C,et al.S-nitrosylation/denitrosylation as a regulatory mechanism of salt stress sensing in sunflower seedlings[J].Physiologia Plantarum,2018,162(1):49-72.
[29] 初 丛.烟草属亚硝基谷胱甘肽还原酶在调控细胞死亡中作用的研究[D].金华:浙江师范大学,2015.
[30] Shi Y F,Wang D L,Wang C,et al.Loss of GSNOR1 function leads to compromised auxin signaling and polar auxin transport[J].Molecular Plant,2015,8(9):1350-1365.
[31] 刘晓庆,徐照龙,许 玲,等.大豆GmNAC8基因克隆与原核表达[J].江苏农业学报,2013,29(4):734-737.